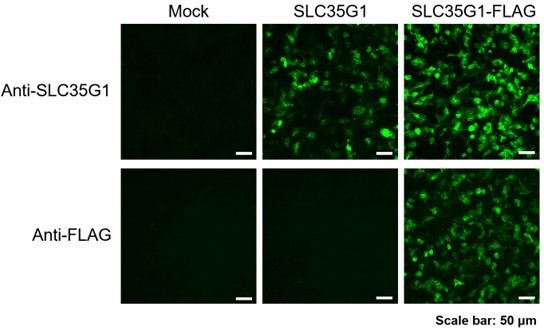
SLC35G1 is a highly chloride-sensitive transporter responsible for the basolateral membrane transport in intestinal citrate absorption
Figures
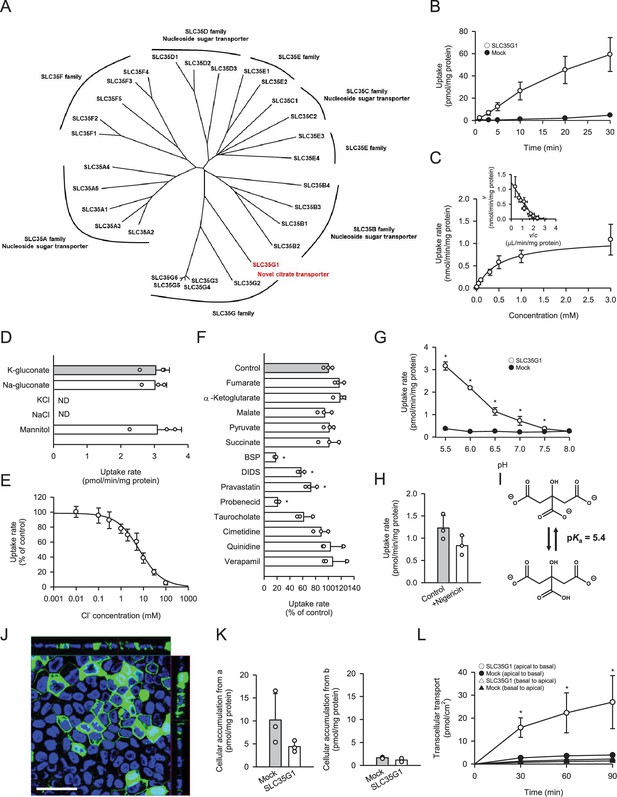
Functional characteristics of SLC35G1 stably expressed in MDCKII cells.
(A) Phylogenetic tree of the SLC35 members and identified functions. (B) Time course of [14C]citrate (1 μM) uptake in SLC35G1-transfected MDCKII cells (open circles) and mock cells (closed circles), evaluated at pH 5.5 and 37°C under the Cl--free condition. (C) Concentration-dependent [14C]citrate uptake by SLC35G1, evaluated at various concentrations for 10 min at pH 5.5 and 37°C under the Cl--free condition. The estimated values of Vmax and Km were 1.11±0.16 nmol/min/mg protein and 519±86 μM, respectively. (D) Effect of ionic conditions on [14C]citrate (1 μM) uptake by SLC35G1, evaluated for 10 min at pH 5.5 and 37°C. K-gluconate in the control uptake solution was replaced as indicated. (E) Concentration-dependent chloride inhibition of [14C]citrate (1 μM) uptake by SLC35G1, evaluated at various chloride concentrations for 10 min at pH 5.5 and 37°C. The estimated value of IC50 was 6.7±1.4 mM. (F) Effect of various compounds on [14C]citrate (1 μM) uptake by SLC35G1, evaluated for 10 min at pH 5.5 and 37°C in the presence (200 μM) or absence (control) of a test compound under the Cl--free condition. BSP, bromosulfophthalein; DIDS, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid. (G) Effect of pH on [14C]citrate (1 μM) uptake in SLC35G1-transfected MDCKII cells (open circles) and mock cells (closed circles), evaluated for 10 min at 37 °C under the Cl--free condition, using the uptake solutions supplemented with 10 mM MES (pH 6.5 and below) or 10 mM HEPES (pH 7.0 and above). (H) Effect of the protonophore nigericin on [14C]citrate (1 μM) uptake by SLC35G1, evaluated for 10 min at pH 5.5 and 37°C under the Cl--free condition in the presence (10 μM) or absence (control) of nigericin after pretreatment for 5 min with or without nigericin under the same conditions. (I) Structural formula illustrating the chemical equilibrium of citrate. (J) Cellular localization of SLC35G1 stably expressed in polarized MDCKII cells. The confocal laser-scanning microscopic image shows SLC35G1 (green) and nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 50 μm. (K) Intracellular accumulation of citrate in polarized MDCKII cells stably expressing SLC35G1 and mock cells on Transwell filters. Intracellular accumulation of [14C]citrate (1 μM) was evaluated for 90 min at pH 5.5 on the apical side (a) and pH 7.4 on the basolateral side (b) in the presence of Cl- (144 mM) on both sides of intestinal epithelial cells. (L) Time course of transcellular [14C]citrate (1 μM) transport in polarized MDCKII cells stably expressing SLC35G1 and mock cells on Transwell filters, evaluated at pH 5.5 in the apical chamber and pH 7.4 in the basolateral chamber in the presence of Cl- (144 mM) in both chambers. Data represent the mean ± SD of three biological replicates (B–H, K, L). Statistical differences were assessed using analysis of variance (ANOVA) followed by Dunnett’s test (D, F, L) or using Student’s t-test (G, H, K). *p<0.05 compared with the control (F), or compared with the mock at each pH (G) or time point (L).
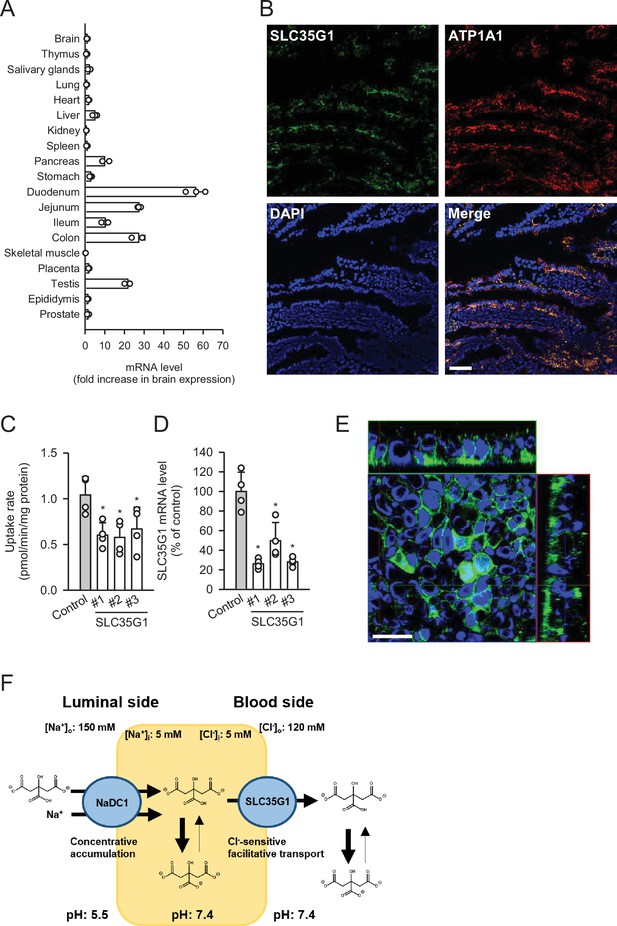
Role of SLC35G1 in intestinal citrate absorption.
(A) Analysis of SLC35G1 mRNA expression in various human tissues using quantitative real-time PCR. (B) The immunofluorescent images show the subcellular localization of SLC35G1 (green), ATP1A1 (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue) in the human jejunum. Scale bar, 100 μm. (C–D) Effect of SLC35G1 silencing on citrate uptake and on SLC35G1 mRNA expression in Caco-2 cells. (C) The uptake rate of [14C]citrate (1 μM) was evaluated for 10 min at pH 5.5 and 37°C under the Cl--free condition in Caco-2 cells transfected with three different siRNAs specific to SLC35G1 mRNA or control siRNA. (D) SLC35G1 mRNA levels were assessed using quantitative real-time PCR analysis. (E) Cellular localization of endogenous SLC35G1 in polarized Caco-2 cells. The confocal laser-scanning microscopic image shows SLC35G1 (green) and nuclei stained with DAPI (blue). Scale bar, 50 μm. (F) Schematic model showing intestinal citrate absorption mediated by NaDC1 and SLC35G1. Data represent the mean ± SD of three technical repeats (A) or biological repeats (C–D). Statistical differences were assessed using analysis of variance (ANOVA) followed by Dunnett’s test (C–D).
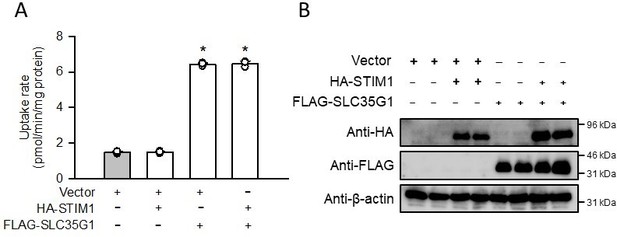
(A) Effect of the coexpression of HA-tagged STIM1 on [14C]citrate (1 μM) uptake by FLAG-tagged SLC35G1 transiently expressed in HEK293 cells.
The uptake was evaluated for 10 min at pH 5.5 and 37°C. Data represent the mean ± SD of three biological replicates. Statistical differences were assessed using ANOVA followed by Dunnett’s test. *, p < 0.05 compared with the control (gray bar). (B) Western blot analysis was conducted by probing for the HA and FLAG tags, using the whole-cell lysate samples (10 µg protein aliquots) prepared from cells expressing HA-STIM1 and/or FLAG-SLC35G1. The blots of β-actin are shown for reference.