Syngeneic natural killer cell therapy activates dendritic and T cells in metastatic lungs and effectively treats low-burden metastases
Figures
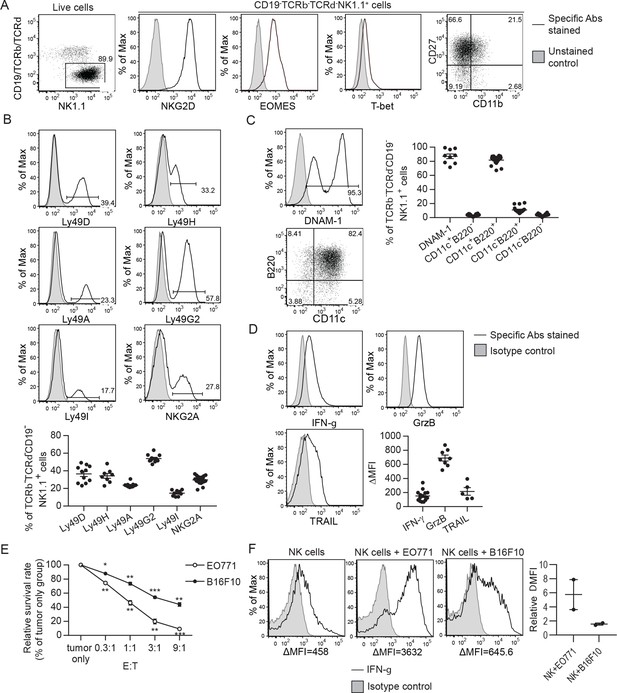
Characterization of ex vivo-expanded murine NK cells.
-
Figure 1—source data 1
Expression of indicated NKRs, activation markers and anti-tumor effectors and in vitro cytotoxicity and IFN-g production in response to tumor cells by the expanded NK cells.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig1-data1-v1.xlsx
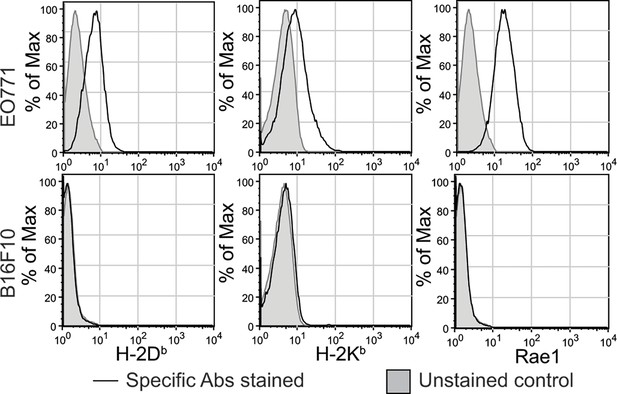
Expression of MHC-I molecules and Rae-1 by EO771 and B16F10 cells in vitro (related to Figure 1E).
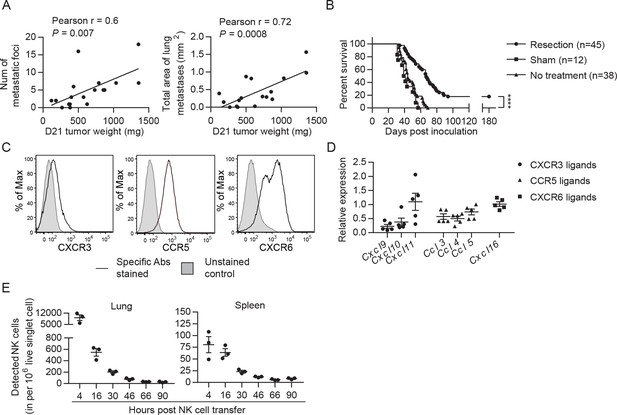
EO771 tumor resection and metastasis model for NK cell treatment.
(A) Positive correlation between primary tumor weight and lung metastasis. Pearson correlations between day 21 tumor weights and the number and total area of metastatic foci on the lung surface are shown. Each dot represents a mouse. (B) Survival of mice who received day 21 tumor resection, sham surgery, or no treatment. Mouse survival was analyzed by means of the Kaplan-Meier estimator and a Log-Rank test: ****p<0.0001. (C) Expanded NK cells express the chemokine receptors CXCR3, CCR5, and CXCR6. Representative flow plots of 10 independent cultures are shown. (D) Expression of CXCR3, CCR5, and CXCR6 ligands in the lung. Specific gene expression was determined by quantitative real-time PCR using lung RNA of day 21 tumor-resected mice collected on day 24 post tumor inoculation and normalized to the expression of cyclophilin a. Each symbol represents a mouse and means ± SEM are shown. (E) Expanded eGFP+ NK cells were transferred into day 21 EO771-resected mice on day 24, and quantified in the single cell suspensions of spleen and lung at the indicated times using FACS. Numbers of eGFP+ NK cells detected in each organ are shown. Each dot represents a mouse (means ± SEM).
-
Figure 2—source data 1
Correlation between tumor weight and lung metastasis, mice survival, expression of CXCR3, CCR5 and CXCR6 ligands, and the number of transferred EGFP+ NK cells.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig2-data1-v1.xlsx
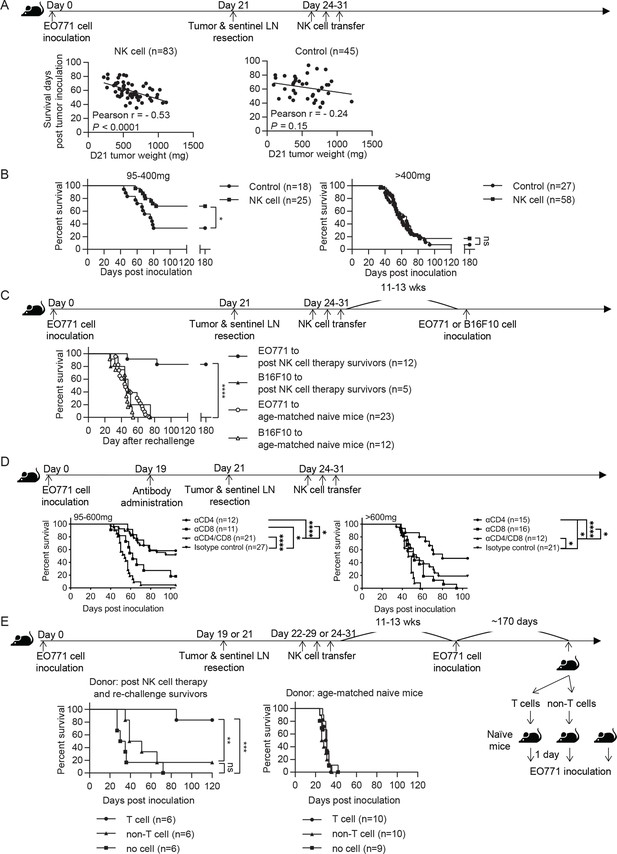
Syngeneic NK cell therapy is effective in treating mice with low metastatic burden in a CD8+-T-cell-dependent manner.
(A) Day 21 tumor weight is inversely correlated with survival duration of NK-cell-treated tumor-resected mice. After tumor and sentinel LN resection on day 21, mice received either sorted NK cells (NK cell) or PBS (Control) at the indicated time-points. The correlation between day 21 tumor weight and survival time of mice was evaluated by Pearson correlation. Each dot represents a mouse. The results have been compiled from five to eight independent experiments. The Control group is the same dataset as presented for the Resection group in Figure 2B. (B) NK cell therapy promotes long-term survival of mice who had light tumors on day 21. The mice described in (A) were separated into 95–400 mg and >400 mg day 21 tumor weight groups for survival analysis by means of the Kaplan-Meier estimator and a Log-Rank test: *p<0.05. (C) NK cell therapy induces tumor-specific protection. Surviving mice after NK cell therapy were re-challenged with EO771 (●, compiled from four independent experiments) or B16F10 cells (▲, compiled from two independent experiments) at 11–13 weeks post therapy, and then monitored for survival. Age-matched naïve mice were inoculated with EO771 (○, compiled from four independent experiments) or B16F10 cells (△, compiled from three independent experiments) as controls. Mouse survival, using 2000 mm3 tumor volume as a cut-off, was analyzed by means of the Kaplan-Meier estimator and a Log-Rank test: ****p<0.001. (D) Depletion of CD4+ or/and CD8+ cells alter the effects of NK cell therapy. Mice were treated with indicated antibodies at day 19 post tumor inoculation, and then received resection followed by NK cell therapy. Mice were grouped according to indicated primary tumor weight for survival analysis by means of the Kaplan-Meier estimator and a Log-Rank test: ns, not significant; *p<0.05; ***p<0.001; ****p<0.0001. The data are compiled from two to three experiments. (E) T cells isolated from surviving mice post NK cell therapy and tumor re-challenge confer anti-tumor activity. Post NK cell therapy and re-challenge survivors were collected from three independent experiments. T cells and non-T cells isolated from each survivor were transferred separately into naïve recipient mice in a 1-donor-to-1-recipient manner, whereas other naïve mice that received no cells served as a control (compiled from two independent experiments). T cells and non-T cells were also isolated from age-matched naïve donors and transferred separately into naïve recipient mice, whereas other naïve mice that received no cells served as a control (compiled from two independent experiments). The recipient mice were then inoculated with EO771 cells one day after cell transfer. Mouse survival, using 2000 mm3 tumor volume as a cut-off, was analyzed by means of the Kaplan-Meier estimator and a Log-rank test: ns, not significant; **p<0.01; ***p<0.001.
-
Figure 3—source data 1
Correlation between tumor weight and survival and mice survival analyses.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig3-data1-v1.xlsx
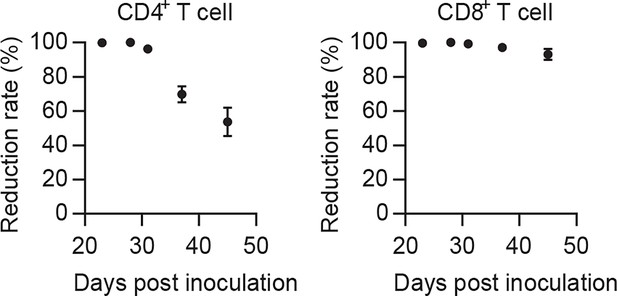
Effectiveness of in vivo depletion of CD4+ and CD8+ T cells by antibody (related to Figure 3D).
-
Figure 3—figure supplement 1—source data 1
Reduction rate of CD4+ and CD8+ T cells after antibody treatment.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig3-figsupp1-data1-v1.xlsx
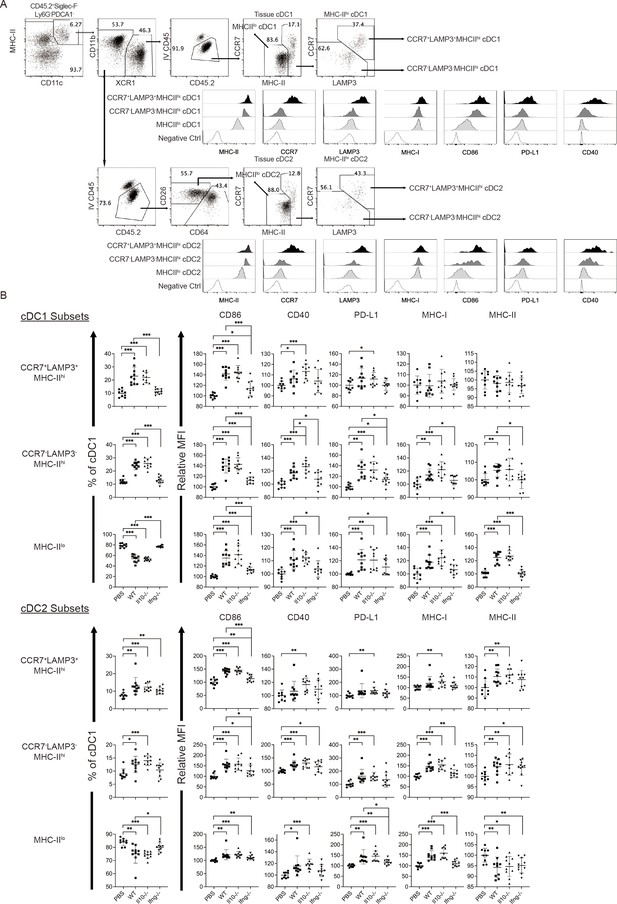
Syngeneic NK cell transfer modulates APC function of cDC in the metastatic lungs.
-
Figure 4—source data 1
Composition of cDC1 and cDC2 subsets and the expression of CD86, CD40, PD-L1, MHC-I and MHC-II by the cDC subsets.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig4-data1-v1.xlsx
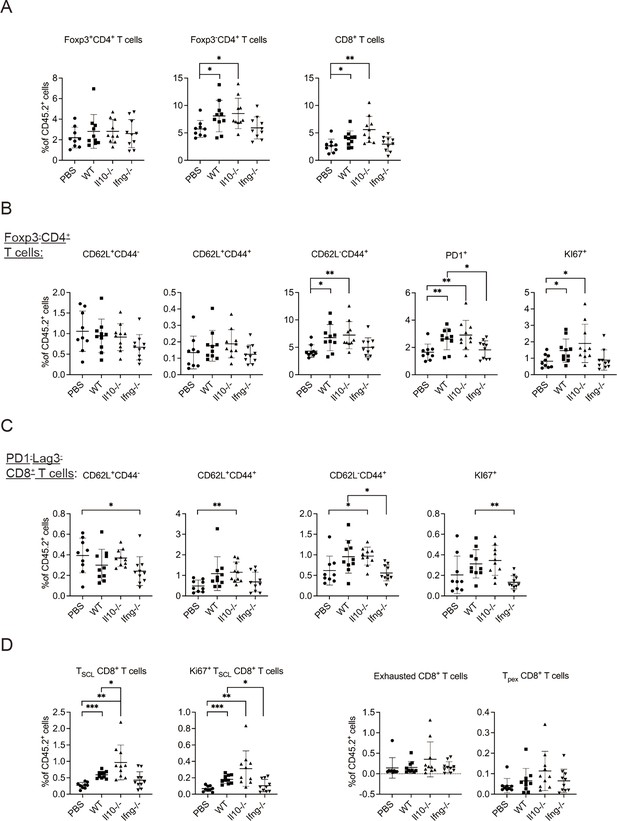
Syngeneic NK cell transfer promotes T cell activation in metastatic lungs.
-
Figure 5—source data 1
The percentages of indicated T cell subpopulations in CD45+ cells.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig5-data1-v1.xlsx
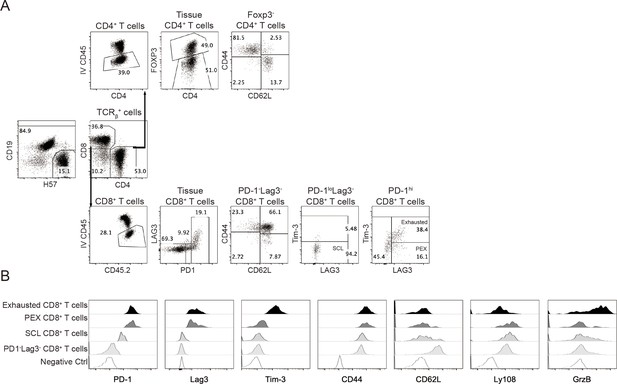
Analysis of T cells in lung tissue (related to Figure 5).
(A) Representative flow plots show the gating of CD4+ and CD8+ T cell subsets. (B) Histograms show the expression of indicated molecules that mark the four CD8+ T cell subsets from a representative NK-cell-treated mouse. The negative controls are either FMO or single stain of a different molecule.
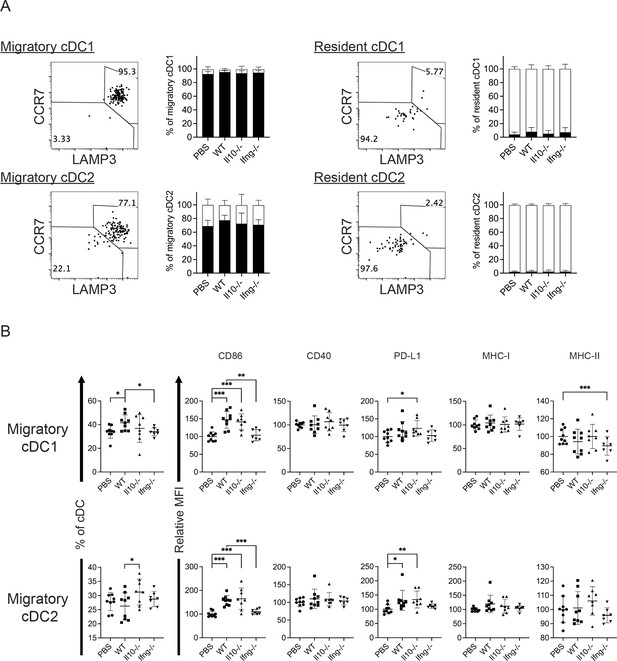
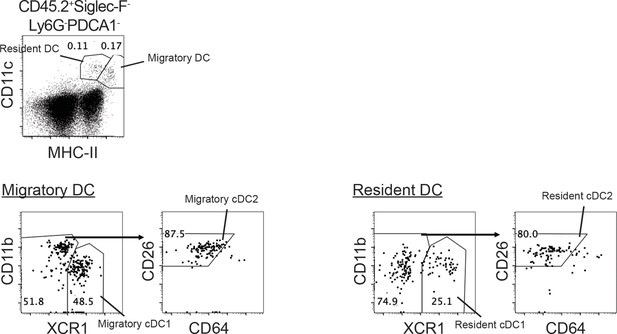
Syngeneic NK cell transfer affects migratory cDCs in mLN.
-
Figure 6—source data 1
The expression of CCR7, LAMP3, CD86, CD40, PD-L1, MHC-I and MHC-II by indicated cDC subsets.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig6-data1-v1.xlsx

Ex vivo-expanded human NK cells exhibit anti-tumor activities in vitro.
(A) Analysis of expanded human NK cells. Live cells among total cells obtained from human PBMC culture (as described in Methods) were analyzed for the expression of indicated molecules by FACS. Representative plots of 33 healthy donors are shown. (B) Expanded human NK cells kill tumor cells in vitro. Sorted HLA-DR+ NK cells from two healthy donors were co-cultured with CFSE-labeled tumor cells at the indicated E:T ratio. Percentage of dead tumor cells represents the mean ± SEM of PI+ cells among CFSE+ tumor cells from triplicate wells. (C) Expanded human NK cells up-regulate IFN-γ production in response to tumor cells in vitro. Sorted HLA-DR+ NK cells from two healthy donors were co-cultured with tumor cells at a 1:1 ratio for 5 hr. Levels of IFN-γ in NK cells were examined by intracellular staining and are calculated as the ∆MFI ± SEM between specific antibody-stained and isotype control antibody-stained samples. Statistical significance was determined by unpaired two-tailed Student’s t-test: *p<0.05, **p<0.01, ***p<0.001 relative to NK cells only.
-
Figure 7—source data 1
The cytotoxicity and IFN-g production by expanded human NK cells in response to tumor cells in vitro.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig7-data1-v1.xlsx
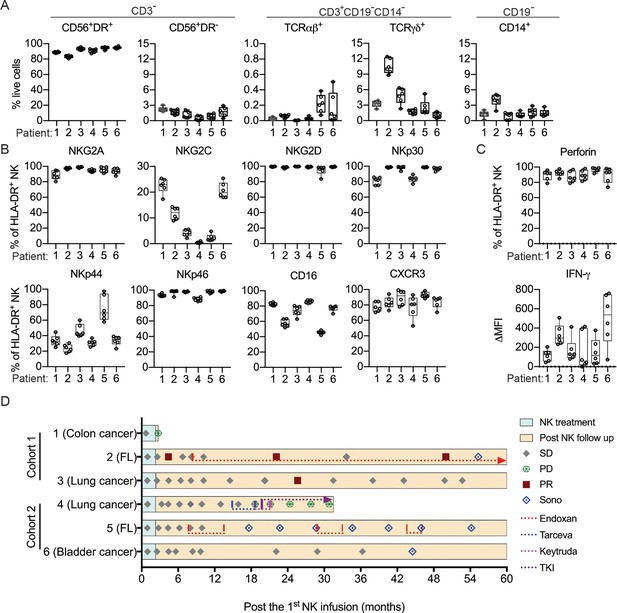
Clinical outcomes of autologous NK cell therapy.
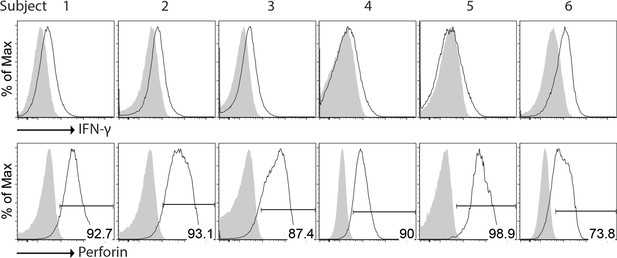
(A) Composition of the infused cells for each patient over six batches of cell preparation. Expression of (B) NKRs, CD16 and CXCR3 and (C) perforin and IFN-γ by the expanded HLA-DR+ NK cells over six batches of cell preparation. Each dot represents a batch of cell preparation. (D) Clinical responses and survival of the six patients. The blue and yellow blocks represent the durations of NK cell treatment and subsequent follow-up, respectively. The response of patients (SD, PD, or PR) was evaluated by CT imaging according to RECIST 1.1. Sono symbol marks follow-up using ultrasound imaging. Colored dotted lines indicate the timeframes for additional medications.
-
Figure 8—source data 1
Composition and expression of NKRs, CD16, CXCR3, perforin and IFN-g by the expanded human NK cells.
- https://cdn.elifesciences.org/articles/99010/elife-99010-fig8-data1-v1.xlsx
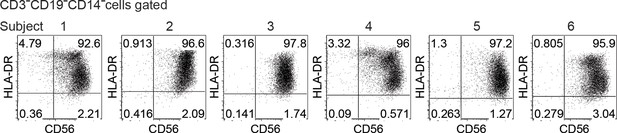
Expression of CD56 and HLA-DR by CD3-CD19-CD14- cells after ex vivo expansion (related to Figure 8A).
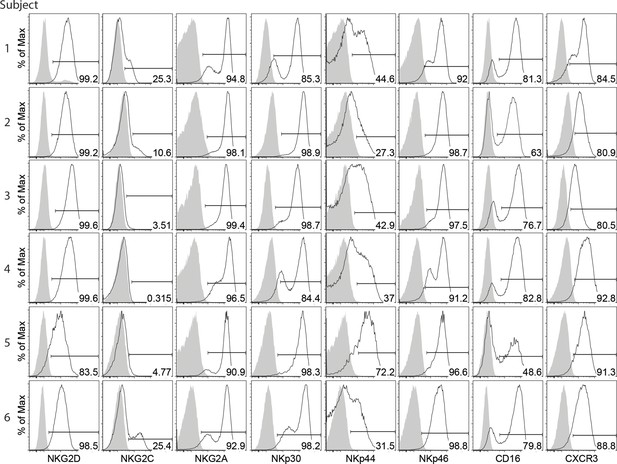
Expression of NKRs, CD16 and CXCR3 by the expanded HLA-DR+ NK cells (related to Figure 8B).
Expression of IFN-γ and perforin in the expanded HLA-DR+ NK cells (related to Figure 8C).
Tables
Baseline characteristics of the patients enrolled in the phase I trial.
| Characteristic | Cohort 1 | Cohort 2 | ||||
|---|---|---|---|---|---|---|
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
| Age | 63 | 42 | 59 | 66 | 54 | 65 |
| Gender | Male | Female | Female | Female | Female | Male |
| Primary cancer | Colon | Follicular lymphoma | Lung | Lung | Follicular lymphoma | Bladder |
| Metastasis | Adrenal Lung Pelvis | Lung | Brain | Bone | ||
| Previous therapies for cancer | ||||||
| Surgery | Yes | No | Yes | Yes | No | Yes |
| Radiotherapy | No | No | No | Yes | No | No |
| Lines of chemo- or targeted therapy | 1 | 3 | 0 | 3 | 2 | 4 |
| Immunotherapy | No | No | No | No | No | Keytruda* |
| ECOG performance status | 0 | 0 | 0 | 0 | 0 | 0 |
| Target lesion (mm) | Pelvic LN (16.3) | Left inguinal LN (18.4) | Mediastinal LN (12.3) | Lung left upper lobe (6.4) | Left axillary LN (10.4) | Pelvic LN (22.7) |
-
*
Ended 7 months before NK cell therapy.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-human CD14 APC/Cyanine7 (Mouse monoclonal) | BioLegend | Cat#:301820 RRID:AB_493694 | (1:50) |
| Antibody | Anti-human CD159 PE (Mouse monoclonal) | Beckman Coulter | Cat#:IM3291U | (1:25) |
| Antibody | Anti-human CD16 eFluor 450 (Mouse monoclonal) | eBioscience | Cat#:48-0168-42 RRID:AB_1272052 | (1:50) |
| Antibody | Anti-human CD183 Alexa Fluor 647 (Mouse monoclonal) | BioLegend | Cat#:353712 RRID:AB_10962946 | (1:10) |
| Antibody | Anti-human CD19 APC-eFluor 780 (Mouse monoclonal) | eBioscience | Cat#:47-0199-42 RRID:AB_1582230 | (1:25) |
| Antibody | Anti-human CD3 APC/Cyanine7 (Mouse monoclonal) | BioLegend | Cat#:300426 RRID:AB_830754 | (1:50) |
| Antibody | Anti-human CD3 eFluor 450 (Mouse monoclonal) | eBioscience | Cat#:48-0038-42 RRID:AB_1518798 | (1:50) |
| Antibody | Anti-human CD314 Brilliant Violet 421 (Mouse monoclonal) | BioLegend | Cat#:320822 RRID:AB_2566510 | (1:10) |
| Antibody | Anti-human CD335 PE (Mouse monoclonal) | BioLegend | Cat#:331908 RRID:AB_1027679 | (1:10) |
| Antibody | Anti-human CD336 PE (Mouse monoclonal) | BioLegend | Cat#:3325108 RRID:AB_756099 | (1:80) |
| Antibody | Anti-human CD337 Alexa Fluor 647 (Mouse monoclonal) | BioLegend | Cat#:325212 RRID:AB_2149448 | (1:20) |
| Antibody | Anti-human CD56 PE-Cyanine7 (Mouse monoclonal) | Invitrogen | Cat#:25-0567-42 RRID:AB_11041529 | (1:25) |
| Antibody | Anti-human HLA-DR FITC (Mouse monoclonal) | BioLegend | Cat#:307632 RRID:AB_1089142 | (1:100) |
| Antibody | Anti-human HLA-DR eFluor 450 (Mouse monoclonal) | eBioscience | Cat#:48-9952-42 RRID:AB_1603291 | (1:50) |
| Antibody | Anti-human IFN-g Alexa Fluor 647 (Mouse monoclonal) | BioLegend | Cat#:502516 RRID:AB_493031 | (1:20) |
| Antibody | Anti-human NKG2C APC (Mouse monoclonal) | R&D Systems | Cat#:FAB138A | (1:10) |
| Antibody | Anti-human Perforin PE (Mouse monoclonal) | eBioscience | Cat#:12-9994-42 RRID:AB_10854416 | (1:50) |
| Antibody | Anti-mouse/human CD45R/B220 APC/Cyanine7 (Rat monoclonal) | BioLegend | Cat#:103224 RRID:AB_313006 | (1:100) |
| Antibody | Anti-mouse CCR5 PE (Mouse monoclonal) | eBioscience | Cat#:12-1951-82 RRID:AB_657684 | (1:100) |
| Antibody | Anti-mouse CCR7 PE (Rat monoclonal) | BioLegend | Cat#:120106 RRID:AB_389357 | (1:50) |
| Antibody | Anti-mouse CCR7 Alexa Fluor 647 (Rat monoclonal) | BioLegend | Cat#:120109 RRID:AB_389235 | (1:50) |
| Antibody | Anti-mouse CD11b BUV805 (Rat monoclonal) | BD Biosciences | Cat#:741934 | (1:400) |
| Antibody | Anti-mouse/human CD11b PerCP/Cyanine5.5 (Rat monoclonal) | BioLegend | Cat#:101228 RRID:AB_893232 | (1:400) |
| Antibody | Anti-mouse CD11c PE/Cyanine5 (Armenian Hamster monoclonal) | BioLegend | Cat#:117316 RRID:AB_493566 | (1:100) |
| Antibody | Anti-mouse CD11c PE (Armenian Hamster monoclonal) | BioLegend | Cat#:117308 RRID:AB_313776 | (1:200) |
| Antibody | Anti-mouse CD11c Super Bright 600 (Armenian Hamster monoclonal) | eBioscience | Cat#:63-0114-82 RRID:AB_2722930 | (1:100) |
| Antibody | Anti-mouse CD11c Super Bright 645 (Armenian Hamster monoclonal) | eBioscience | Cat#:64-0114-82 RRID:AB_2717081 | (1:100) |
| Antibody | Anti-mouse CD19 APC/Fire 810 (Rat monoclonal) | BioLegend | Cat#:115578 RRID:AB_2892274 | (1:400) |
| Antibody | Anti-mouse CD19 FITC (Rat monoclonal) | BioLegend | Cat#:115506 RRID:AB_313640 | (1:400) |
| Antibody | Anti-mouse CD19 Alexa Fluor 700 (Rat monoclonal) | BioLegend | Cat#:115528 RRID:AB_493734 | (1:1600) |
| Antibody | Anti-mouse CD19 Brilliant Ultra Violet 615 (Rat monoclonal) | eBioscience | Cat#:366-0193-82 RRID:AB_2925404 | (1:100) |
| Antibody | Anti-mouse CD26 BUV661 (Rat monoclonal) | BD | Cat#:741492 | (1:100) |
| Antibody | Anti-mouse/rat/humanCD27 PE/Cyanine7 (Armenian Hamster monoclonal) | BioLegend | Cat#:124216 RRID:AB_10639726 | (1:100) |
| Antibody | Anti-mouse CD4 APC/Fire 810 (Rat monoclonal) | BioLegend | Cat#:100480 RRID:AB_2860583 | (1:400) |
| Antibody | Anti-mouse CD40 PE/Cyanine7 (Rat monoclonal) | BioLegend | Cat#:124622 RRID:AB_10897812 | (1:50) |
| Antibody | Anti-mouse CD44 BUV563 (Rat monoclonal) | BD Biosciences | Cat#:741227 | (1:100) |
| Antibody | Anti-mouse/human CD44 Brilliant Violet 510 (Rat monoclonal) | BioLegend | Cat#:103044 RRID:AB_2561391 | (1:80) |
| Antibody | Anti-mouse CD45 FITC (Rat monoclonal) | BioLegend | Cat#:103108 RRID:AB_312972 | 3 ug/mouse |
| Antibody | Anti-mouse CD45.2 BUV395 (mouse monoclonal) | BD Biosciences | Cat#:564616 | (1:100) |
| Antibody | Anti-mouse CD62L BV711 (Rat monoclonal) | BD Biosciences | Cat#:740660 | (1:800) |
| Antibody | Anti-mouse CD63 Alexa Fluor 647 (Rat monoclonal) | BioLegend | Cat#:143922 RRID:AB_2832513 | (1:100) |
| Antibody | Anti-mouse CD64 PE/Dazzle 594 (mouse monoclonal) | BioLegend | Cat#:139320 RRID:AB_2566558 | (1:50) |
| Antibody | Anti-mouse CD64 PE (mouse monoclonal) | BioLegend | Cat#:139304 RRID:AB_10613467 | (1:50) |
| Antibody | Anti-mouse CD80 PE-Cyanine5 (Armenian Hamster monoclonal) | eBioscience | Cat#:15-0801-82 RRID:AB_468774 | (1:100) |
| Antibody | Anti-mouse CD86 BUV737 (Rat monoclonal) | BD Biosciences | Cat#:741737 | (1:100) |
| Antibody | Anti-mouse CD86 PE/Dazzle 594 (Rat monoclonal) | BioLegend | Cat#:105042 RRID:AB_2566409 | (1:200) |
| Antibody | Anti-mouse CD8a Brilliant Violet 570 (Rat monoclonal) | BioLegend | Cat#:100740 RRID:AB_10897645 | (1:200) |
| Antibody | Anti-mouse CD8a Brilliant Ultra Violet 563 (Rat monoclonal) | eBioscience | Cat#:365-0081-82 RRID:AB_2920971 | (1:100) |
| Antibody | Anti-mouse CD8a Super Bright 645 (Rat monoclonal) | eBioscience | Cat#:64-0081-82 RRID:AB_2662353 | (1:100) |
| Antibody | Anti-mouse CXCR3 PE (Armenian Hamster monoclonal) | eBioscience | Cat#:12-1831-82 RRID:AB_1210734 | (1:100) |
| Antibody | Anti-mouse CXCR6 PE (Rat monoclonal) | BioLegend | Cat#:151103 RRID:AB_2566545 | (1:100) |
| Antibody | anti-mouse DNAM-1 PE (Rat monoclonal) | BioLegend | Cat#:128806 RRID:AB_1186119 | (1:100) |
| Antibody | Anti-EOMES PerCP-eFluor 710 (Rat monoclonal) | eBioscience | Cat#:46-4877-42 RRID:AB_2573759 | (1:50) |
| Antibody | Anti-mouse Foxp3 PE-Cyanine5 (Rat monoclonal) | eBioscience | Cat#:15-5773-82 RRID:AB_468806 | (1:100) |
| Antibody | Anti-human/mouse Granzyme B Pacific Blue (Mouse monoclonal) | BioLegend | Cat#:515408 RRID:AB_2562195 | (1:50) |
| Antibody | Anti-human/mouse Granzyme B PE (Mouse monoclonal) | BioLegend | Cat#:372208 RRID:AB_2687031 | (1:50) |
| Antibody | Anti-mouse H-2 Class I BV750 (Rat monoclonal) | BD Biosciences | Cat#:749712 | (1:400) |
| Antibody | Anti-mouse H-2D b PE (Mouse monoclonal) | BioLegend | Cat#:111508 RRID:AB_313512 | (1:50) |
| Antibody | Anti-mouse H-2Kb APC (Mouse monoclonal) | eBioscience | Cat#:17-5958-82 RRID:AB_1311280 | (1:400) |
| Antibody | Anti-mouse H-2Kb/H2Db Alexa Fluor 647 (Mouse monoclonal) | BioLegend | Cat#:114612 RRID:AB_492931 | (1:100) |
| Antibody | Anti-mouse I-A/I-E BUV496 (Rat monoclonal) | BD Biosciences | Cat#:750281 | (1:100) |
| Antibody | Anti-mouse I-A/I-E BUV615 (Rat monoclonal) | BD Biosciences | Cat#:751570 | (1:400) |
| Antibody | Anti-mouse I-A/I-E PerCP/Cyanine5.5 (Rat monoclonal) | BioLegend | Cat#:107626 RRID:AB_2191071 | (1:400) |
| Antibody | Anti-mouse IFN-g Brilliant Ultra Violet 737 (Rat monoclonal) | eBioscience | Cat#:367-7311-82 RRID:AB_2896044 | (1:50) |
| Antibody | Anti-mouse IFN-g PE (Rat monoclonal) | BioLegend | Cat#:505808 RRID:AB_315401 | (1:100) |
| Antibody | Anti-mouse IL-12/23p40 APC (Rat monoclonal) | BioLegend | Cat#:505206 RRID:AB_315369 | (1:200) |
| Antibody | Anti-mouse Ki67 FITC (Rat monoclonal) | BioLegend | Cat#:652409 RRID:AB_2562140 | (1:50) |
| Antibody | Anti-mouse Lag-3 Brilliant Violet 785 (Rat monoclonal) | BioLegend | Cat#:125219 RRID:AB_2566571 | (1:25) |
| Antibody | Anti-mouse Ly108 BUV661 (Mouse monoclonal) | BD Biosciences | Cat#:741679 RRID:AB_2871064 | (1:200) |
| Antibody | Anti-mouse Ly49A FITC (Rat monoclonal) | BioLegend | Cat#:116805 RRID:AB_313756 | (1:400) |
| Antibody | Anti-mouse Ly49D FITC (Rat monoclonal) | BioLegend | Cat#:138303 RRID:AB_10588709 | (1:1000) |
| Antibody | anti-mouse Ly49G2 FITC (Rat monoclonal) | eBioscience | Cat#:11-5781-82 RRID:AB_763604 | (1:400) |
| Antibody | Anti-mouse Ly49H APC (Mouse monoclonal) | BioLegend | Cat#:144712 RRID:AB_2783111 | (1:400) |
| Antibody | Anti-mouse Ly49I FITC (Mouse monoclonal) | eBioscience | Cat#:11-5895-82 RRID:AB_465301 | (1:100) |
| Antibody | Anti-mouse Ly6C PerCP (Rat monoclonal) | BioLegend | Cat#:128028 RRID:AB_10897805 | (1:200) |
| Antibody | Anti-mouse Ly6G Brilliant Violet 785 (Rat monoclonal) | BioLegend | Cat#:127645 RRID:AB_2566317 | (1:100) |
| Antibody | Mouse IgG1, κκPacific Blue (Mouse monoclonal) | BioLegend | Cat#:400151 RRID:AB_2923473 | Isotype control, same titer used as specific Ab |
| Antibody | Mouse IgG1, PerCP-eFluor 710 (Mouse monoclonal) | eBioscience | Cat#:46-4714-82 RRID:AB_1834453 | Isotype control, same titer used as specific Ab |
| Antibody | Mouse IgG1, κeFluor 660 (Mouse monoclonal) | eBioscience | Cat#:50-4714-82 RRID:AB_10597301 | Isotype control, same titer used as specific Ab |
| Antibody | Anti-mouse NK1.1 PE/Cyanine7 (Mouse monoclonal) | BioLegend | Cat#:108714 RRID:AB_389363 | (1:150) |
| Antibody | Anti-mouse NK1.1 PerCP/Cyanine5.5 (Mouse monoclonal) | BioLegend | Cat#:108728 RRID:AB_2132705 | (1:50) |
| Antibody | Anti-mouse NK1.1 Brilliant Ultra Violet 496 (Mouse monoclonal) | eBioscience | Cat#:364-5941-82 RRID:AB_2925353 | (1:100) |
| Antibody | Anti-mouse NKG2A PerCP-eFluor 710 (Mouse monoclonal) | eBioscience | Cat#:46-5897-82 RRID:AB_2573799 | (1:50) |
| Antibody | Anti-mouse NKG2D PE (Rat monoclonal) | eBioscience | Cat#:12-5882-82 RRID:AB_465996 | (1:50) |
| Antibody | Anti-mouse PD-1 eFluor 450 (Rat monoclonal) | eBioscience | Cat#:48-9981-82 RRID:AB_11150068 | (1:50) |
| Antibody | Anti-mouse PDCA1 Alexa Fluor 700 (Rat monoclonal) | BioLegend | Cat#:127038 RRID:AB_2819861 | (1:400) |
| Antibody | Anti-mouse PDCA1 Brilliant Violet 650 (Rat monoclonal) | BioLegend | Cat#:127019 RRID:AB_2562477 | (1:100) |
| Antibody | Anti-mouse PD-L1 Super Bright 600 (Rat monoclonal) | eBioscience | Cat#:63-5982-82 RRID:AB_2688101 | (1:200) |
| Antibody | Anti-mouse Rae-1 PE (Rat monoclonal) | R&D Systems | Cat#:FAB17582P | (1:100) |
| Antibody | Rat IgG 2 a, κ PE-Cyanine5 (Rat monoclonal) | eBioscience | Cat#:15-4321-82 RRID:AB_470140 | Isotype control, same titer used as specific Ab |
| Antibody | Rat IgG1, κ APC (Rat monoclonal) | BioLegend | Cat#:400412 RRID:AB_326518 | Isotype control, same titer used as specific Ab |
| Antibody | Rat IgG1, κ PE (Rat monoclonal) | BioLegend | Cat#:400408 RRID:AB_326514 | Isotype control, same titer used as specific Ab |
| Antibody | Rat IgG1, κBrilliant Ultra Violet 737 (Rat monoclonal) | eBioscience | Cat#:367-4301-81 RRID:AB_2896005 | Isotype control, same titer used as specific Ab |
| Antibody | Anti-mouse siglecF BV750 (Rat monoclonal) | BD Biosciences | Cat#:747316 | (1:200) |
| Antibody | Anti-mouse siglecF BV510 (Rat monoclonal) | BD Biosciences | Cat#:740158 | (1:100) |
| Antibody | Anti-mouse siglecF Super Bright 780 (Rat monoclonal) | eBioscience | Cat#:78-1702-82 RRID:AB_2744908 | (1:200) |
| Antibody | Anti-human/mouse T-bet eFluor 660 (Mouse monoclonal) | eBioscience | Cat#:50-5825-82 RRID:AB_10596655 | (1:25) |
| Antibody | Anti-mouse TCRb BUV496 (Armenian Hamster monoclonal) | BD Biosciences | Cat#:749915 | (1:50) |
| Antibody | Anti-mouse TCRb PE/Dazzle 594 (Armenian Hamster monoclonal) | BioLegend | Cat#:109240 RRID:AB_2565654 | (1:100) |
| Antibody | Anti-mouse TCRb-FITC (Armenian Hamster monoclonal) | homemade | (1:200) | |
| Antibody | Anti-mouse TCRd (Armenian Hamster monoclonal) | homemade | (1:500) | |
| Antibody | Anti-mouseTim-3 PE/Cyanine7 (Rat monoclonal) | BioLegend | Cat#:119716 RRID:AB_2571932 | (1:200) |
| Antibody | Anti-mouse TRAIL PE (Rat monoclonal) | BioLegend | Cat#:109306 RRID:AB_2205927 | (1:100) |
| Antibody | Anti-mouse/rat XCR1 Brilliant Violet 421 (Mouse monoclonal) | BioLegend | Cat#:148216 RRID:AB_2565230 | (1:100) |
| Antibody | Anti-mouse/rat XCR1Brilliant Violet 785 (Mouse monoclonal) | BioLegend | Cat#:148225 RRID:AB_2783119 | (1:400) |
| Sequence-based reagent | Cxcl9_F | This paper | qRT-PCR primer | GGAGTTCGAGGAACCCTAGTG |
| Sequence-based reagent | Cxcl9_R | This paper | qRT-PCR primer | GGGATTTGTAGTGGATCGTGC |
| Sequence-based reagent | Cxcl10_F | This paper | qRT-PCR primer | CCAAGTGCTGCCGTCATTTTC |
| Sequence-based reagent | Cxcl10_R | This paper | qRT-PCR primer | GGCTCGCAGGGATGATTTCAA |
| Sequence-based reagent | Cxcl11_F | This paper | qRT-PCR primer | GGCTTCCTTATGTTCAAACAGGG |
| Sequence-based reagent | Cxcl11_R | This paper | qRT-PCR primer | GCCGTTACTCGGGTAAATTACA |
| Sequence-based reagent | Ccl3_F | This paper | qRT-PCR primer | TTCTCTGTACCATGACACTCTGC |
| Sequence-based reagent | Ccl3_R | This paper | qRT-PCR primer | CGTGGAATCTTCCGGCTGTAG |
| Sequence-based reagent | Ccl4_F | This paper | qRT-PCR primer | TTCCTGCTGTTTCTCTTACACCT |
| Sequence-based reagent | Ccl4_R | This paper | qRT-PCR primer | CTGTCTGCCTCTTTTGGTCAG |
| Sequence-based reagent | Ccl5_F | This paper | qRT-PCR primer | GCTGCTTTGCCTACCTCTCC |
| Sequence-based reagent | Ccl5_R | This paper | qRT-PCR primer | TCGAGTGACAAACACGACTGC |
| Sequence-based reagent | Cxcl16_F | This paper | qRT-PCR primer | CCTTGTCTCTTGCGTTCTTCC |
| Sequence-based reagent | Cxcl16_R | This paper | qRT-PCR primer | TCCAAAGTACCCTGCGGTATC |
| Sequence-based reagent | Cypa_F | This paper | qRT-PCR primer | GAGCTGTTTGCAGACAAAGTTC |
| Sequence-based reagent | Cypa_R | This paper | qRT-PCR primer | CCCTGGCACATGAATCCTGG |