Elucidating ATP’s role as solubilizer of biomolecular aggregate
Figures
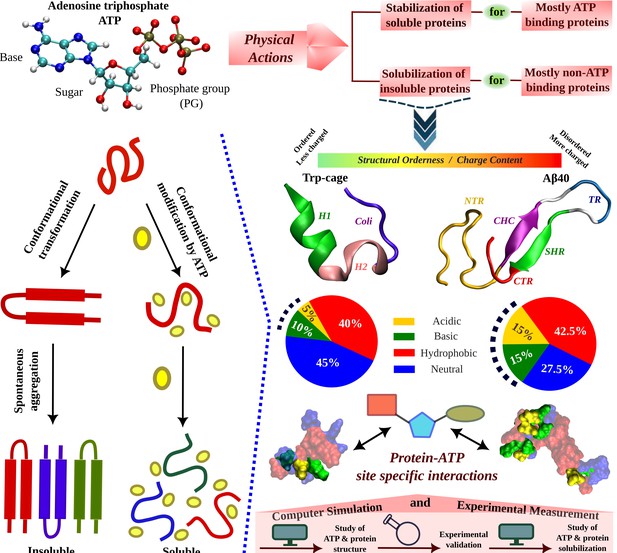
Schematic Representation of ATP's Role in Modulating Protein Aggregation and Conformational Plasticity.
A schematic representation shows protein undergoes spontaneous aggregation in aqueous medium through specific conformational transformation prone to aggregation. Adenosine triphosphate (ATP) can prevent protein aggregation and improves its solubility. ATP’s effect in protein conformational plasticity has been tested for two contrasting protein molecules belonging from two extreme spectrums of the protein family. One is the globular, structurally ordered protein Trp-cage and the other one is intrinsically disordered protein (IDP), Aβ40, containing comparatively more charged residues (according to the nature of typical IDPs). The highly aggregation-prone Aβ40 protein is popularly well known for causing neurodegenerative disorders (Alzheimer’s disease, AD). The structures of both the proteins are shown in the new cartoon representation highlighting the protein region-wise coloration scheme. For Trp-cage the three distinct regions, (1) Helix (H1, 1–9), (2) 3–10 Helix (H2, 10–15), and (3) Coil (coil, 16–20) are shown in green, pink, and navy blue colors, respectively. For Aβ40, the (1) N-terminal region (NTR, 1–16), (2) central hydrophobic core (CHC, 17–21), (3) turn (TR, 24–27), (4) secondary hydrophobic region (SHR, 30–35), and the (5) C-terminal regions (CTR, 36–40) are shown in gold, purple, blue, green, and red colors, respectively. The hydrophobicity index of each of the proteins is shown in pie chart representation containing acidic (gold), basic (green), hydrophobic (red), and neutral (blue) residue content. Each of the proteins shown in the surface model is colored according to the respective hydrophobicity nature. Protein–ATP (base part: red, sugar moiety: cyan, and phosphate group: green) site-specific interactions are tested. The current study of ATP’s effect on protein conformational plasticity is performed combining both simulation and experiment based on computational predictions validated by experimental measurement followed by computational reasoning and correlation of ATP-driven conformational modification to protein aggregation scenario.
-
Figure 1—source data 1
Sequence-based analysis.
- https://cdn.elifesciences.org/articles/99150/elife-99150-fig1-data1-v1.docx
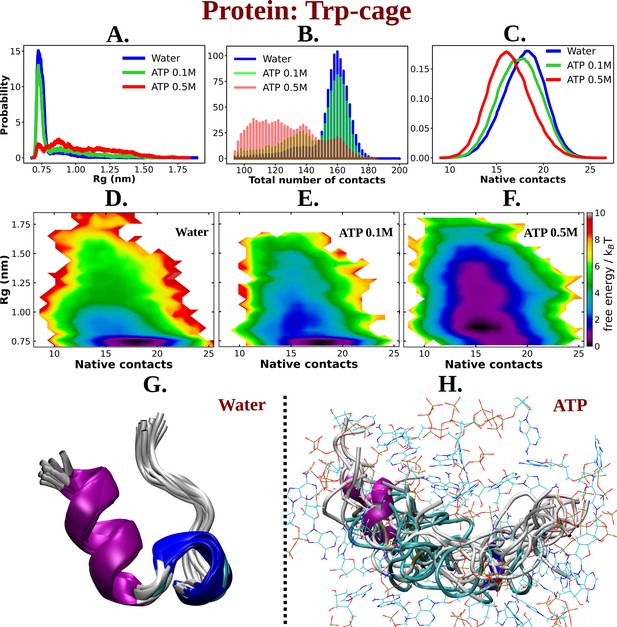
ATP facilitates the chain extension of the globular protein Trp-cage.
(A) The probability distribution of Rg of Trp-cage compared in neat water and in 0.1 and 0.5 M adenosine triphosphate (ATP). (B) Probability of the total number of contact formation among the residues of Trp-cage monomer is compared in absence (water) and presence of ATP (at 0.1 and 0.5 M). The probability distribution of native contacts (nc) of the protein in water and in 0.1 and 0.5 M ATP solutions is being shown in (C). (D), (E), and (F) represent the 2D-free energy profile of Trp-cage corresponding to the Rg and nc of the protein in water, 0.1, and 0.5 M ATP solutions, respectively. Snapshots containing overlay of protein’s conformations in absence of ATP (neat water) and in presence of ATP (0.5 M) are shown in (G) and (H), respectively. Protein is colored by secondary structure and ATP molecules in (H) are shown in line representation with an atom-based coloring scheme (C: cyan, N: blue, O: red).
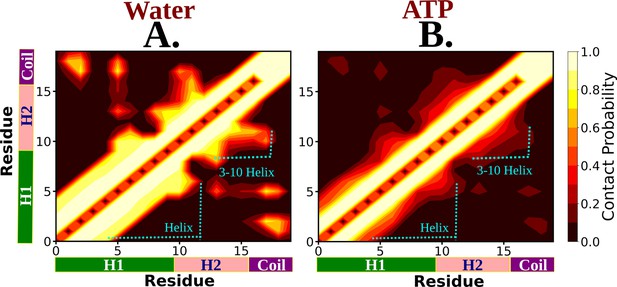
The residue-wise contact map of Trp-cage monomer is shown for neat water and 0.5 M aqueous adenosine triphosphate (ATP) solution in (A) and (B), respectively.
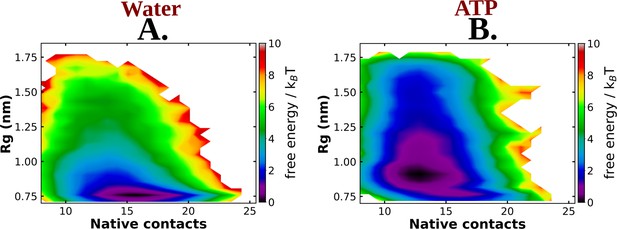
The 2D-free energy profile of Trp-cage monomer estimated with respect to Rg and native contacts are shown in (A) and (B) for Trp-cage in neat water and 0.5 M adenosine triphosphate (ATP), respectively, for the simulations with Charmm36 force field.

Mechanistic understanding of ATP's effect on the conformational modulation of Trp-cage.
Residue-wise total percentage of helix and 3–10 helix content of Trp-cage protein in absence and presence of adenosine triphosphate (ATP) (0.5 M ATP) are shown in (A) and (B), respectively. (C) The solvent-accessible surface area is calculated (with gromacs module of ‘gmx sasa’) for Trp-cage and represented for the aqueous medium without and with ATP corresponding to each of the protein residues in a bar plot representation. (D) shows a representative snapshot of ATP’s (in licorice representation with atom-based coloring scheme) interaction with Trp-cage (new cartoon representation). Green: H1 (1–9), pink: H2 (10–15), navy blue: coil (16–20). (E) Preferential interaction coefficient (Γ) of different parts of ATP: PG, sugar, and base (with respect to solvent, water) with protein is being compared. (F) Bar plot representation of coulombic and LJ interaction performed by all three different parts of ATP (PG, sugar, and base) with Trp-cage. (G) represents the comparative plots of the preferential interaction coefficient (Γ) of ATP with the three structurally different parts (H1, H2, and coil) of Trp-cage. (H) The change in the secondary structure content (helix and 3–10 helix) due to action of ATP is being represented. The difference in helix and 3–10 helix content in neat water from that of ATP solution is shown in bar plots.
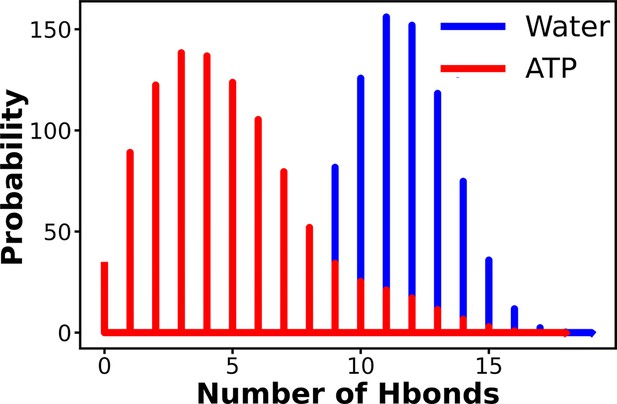
The probability distribution of the number of intra-chain hydrogen bonds of Trp-cage has been compared for neat water and in 0.5 M adenosine triphosphate (ATP) solution.
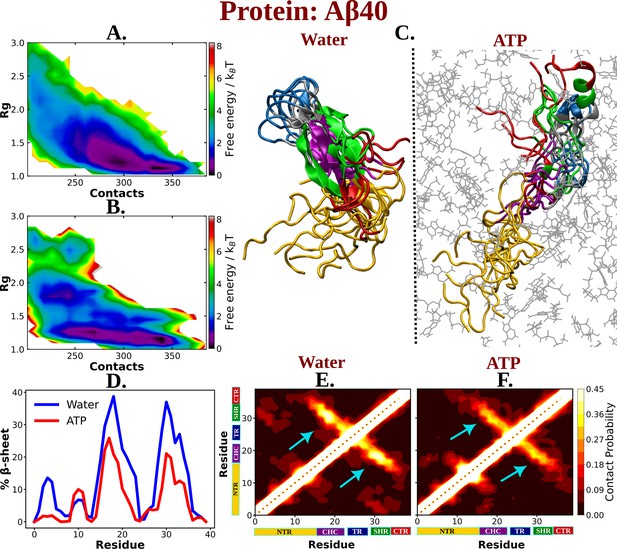
The influence of ATP on the protein chain extension of the IDP Aβ40.
The 2D-free energy profile of Aβ40 monomer estimated with respect to Rg and total number of intra-chain contacts are shown in (A) and (B) for Aβ40 in neat water and 0.5 M adenosine triphosphate (ATP), respectively. (C) compares the simulation snapshots of Aβ40 monomer in neat water and in presence of ATP. Multiple conformations are overlaid for each of the cases to represent the statistical significance. Protein is colored region-wise as done in Figure 1 and ATP molecules are shown by gray color line representation. (D) compares the β-sheet content of the Aβ40 protein in water and in 0.5 M ATP solution. (E) and (F) show the residue-wise intra-chain contact map of Aβ40 in absence and in presence of ATP (0.5 M), respectively.
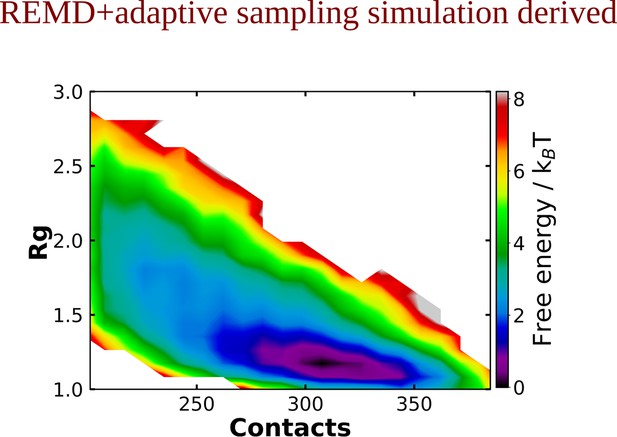
The 2D-free energy profile estimated for Aβ40 protein in absence of adenosine triphosphate (ATP) obtained from replica exchange molecular dynamics (REMD) simulation followed by adaptive sampling simulations.
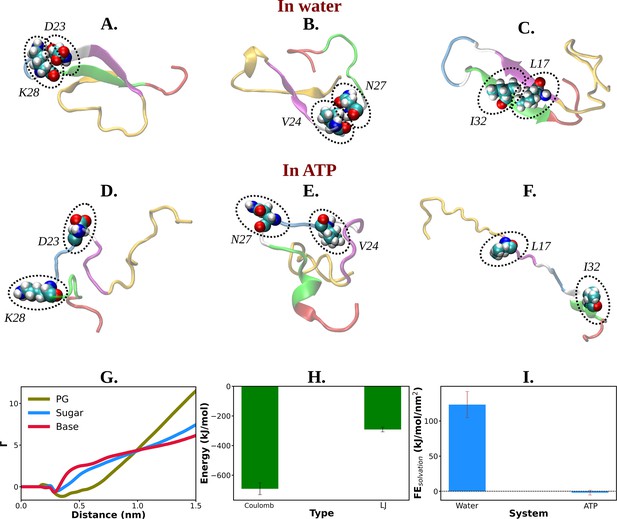
Mechanistic insight into ATP's role in the conformational modulation of abeta40.
(A), (B), and (C) show the representative snapshots of different pairs of interacting residues namely, D23–K28, V24–N27, and L17–I32, respectively, compared for salt water. The similar set of interactions are being represented in (D–F) for adenosine triphosphate (ATP) solution containing salt. (G) Preferential interaction coefficient (Γ) of different parts of ATP (PG, sugar, and base) with protein is being represented with respect to solvent water. (H) The combined coulombic and LJ interaction energies imparted by all the three parts of ATP with Aβ40 are shown. (I) The free energy of solvation (calculated by the gromacs module of ‘gmx sasa’) of Aβ40 protein in absence and in presence of ATP is shown in a bar plot diagram. The vertical lines over the bars show the error bars.
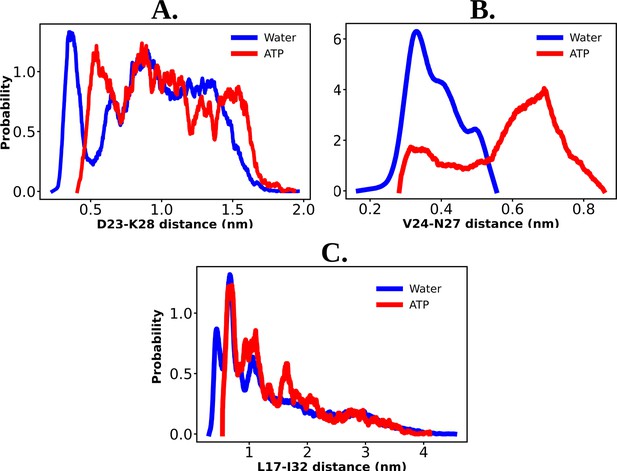
The probability distribution of the distance between the residue pair of D23–K28 (A), V24–N27 (B), and L17–I32 (C) are shown for the protein Aβ40 both in 50 mM NaCl salt solution and 0.5 M aqueous adenosine triphosphate (ATP) solution containing 50 mM NaCl salt.
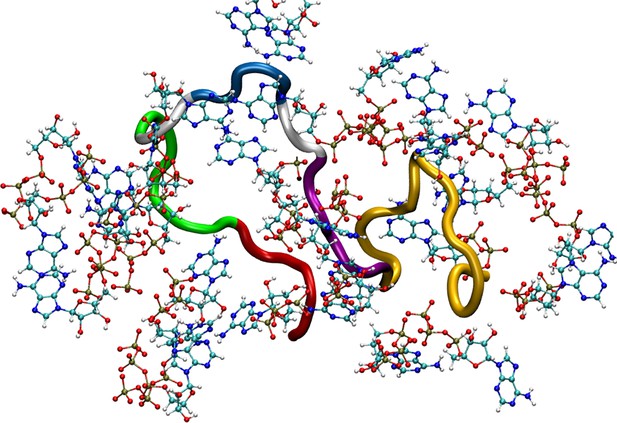
Figure shows a simulation snapshot representing adenosine triphosphate (ATP’s) interaction with the Aβ40 protein.
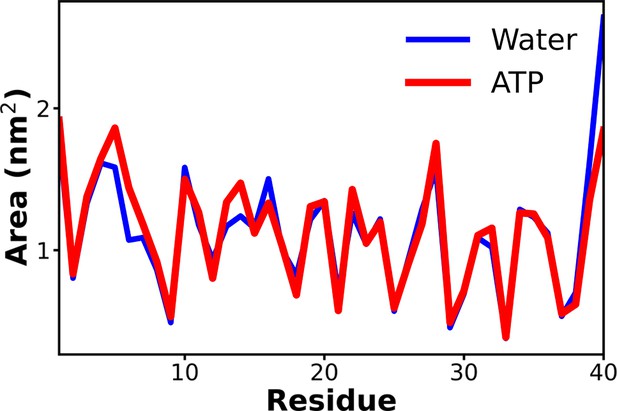
The comparison of the solvent-accessible surface area of the protein Aβ40 has been shown for 50 mM NaCl salt solution (blue) and 0.5 M adenosine triphosphate (ATP) in 50 mM NaCl salt (red).
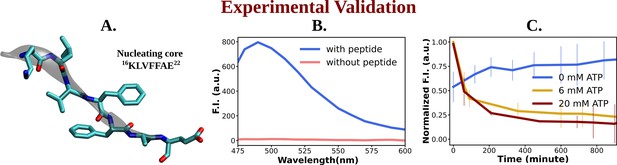
Experimental study of ATP's influence on the aggregation of the nucleating core of Aβ40.
(A) shows the representative snapshot of the peptide belonging to the nucleating core (16th to 22nd residue [Ac-KLVFFAE-NH2, Ac-KE]) of the Aβ40 protein, which is utilized for the experimental measurements. (B) represents emission spectra of Thioflavin T (ThT) in the presence (blue) and absence (pink) of peptide assembly in 10 mM HEPES (4-(2-Hydroxyethyl)piperazine-1-ethane-sulfonic acid) buffer pH 7.2. Excitation wavelength (λex) = 440 nm (final concentration [Ac-KE] = 200 µM, [ThT] = 30 µM). (C) shows a comparative plot of ThT (30 µM) assay of Ac-KE (300 µM) assembly with time in 10 mM pH 7.2 HEPES buffer with 0 mM (blue curve), 6 mM (yellow curve), and 20 mM (dark red curve) of adenosine triphosphate (ATP). The vertical lines over the bars show the error bars. The experiments were replicated on two independent samples.
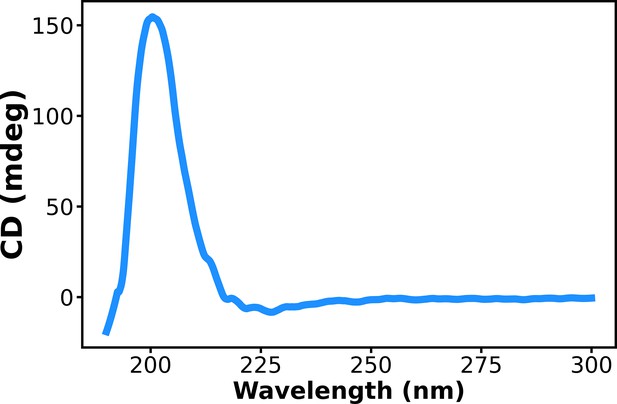
Circular dichroism (CD) spectrum of aged (11–15 days) assembly of Ac-KE.
[Ac-KE] = 200 µM.
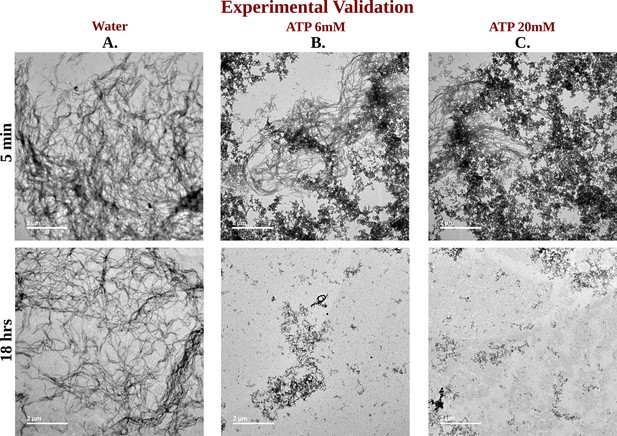
TEM Micrographs of Ac-KE Assemblies: Effects of ATP Concentration Over Time.
Library of TEM micrographs of Ac-KE (300 µM) assemblies in 10 mM pH 7.2 HEPES buffer at 5 min (up) and after 18 hr (down) of incubation, in presence of (A) 0 mM adenosine triphosphate (ATP), (B) 6 mM ATP, and (C) 20 mM ATP are being represented.
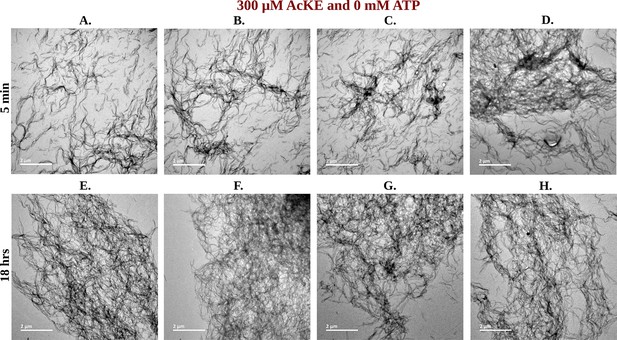
Library of TEM micrographs of 300 µM Ac-KE in 0 mM adenosine triphosphate (ATP) at 5 min (A–D) and 18 hr (E–H).
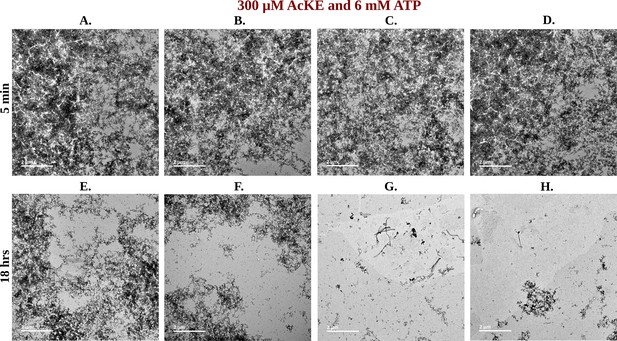
Library of TEM micrographs of 300 µM Ac-KE in 6 mM adenosine triphosphate (ATP) at 5 min (A–D) and 18 hr (E–H).
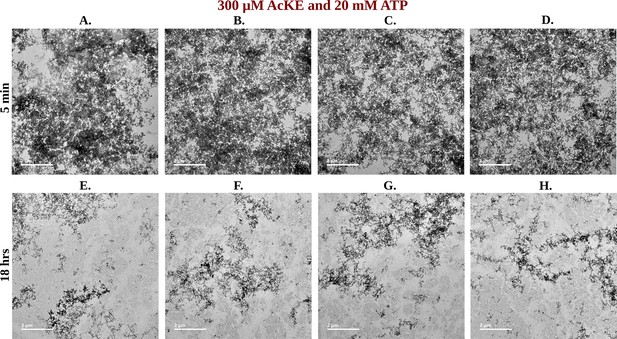
Library of TEM micrographs of 300 µM Ac-KE in 20 mM adenosine triphosphate (ATP) at 5 min (A–D) and 18 hr (E–H).
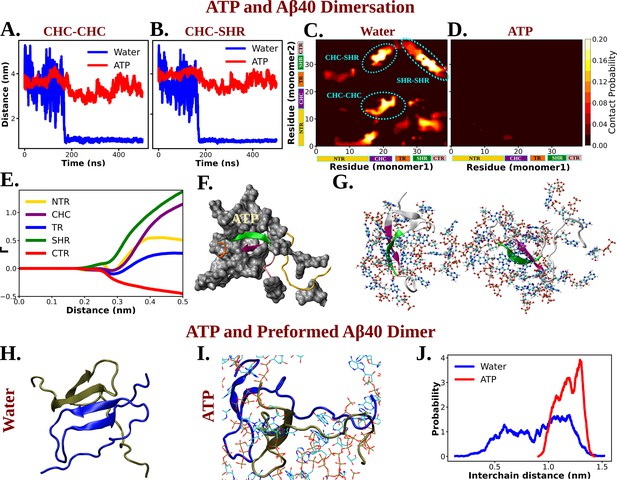
Molecular Basis of ATP's Role in Solubilizing Aβ40 Aggregates.
(A) and (B) show the time profile of distance between the two actively interacting regions of two protein chains namely CHC–CHC and CHC–SHR, respectively, both in neat water and in presence of adenosine triphosphate (ATP) cosolute (0.5 M ATP in 50 mM NaCl solution).(C) and (D) represent residue-wise inter-protein contact map of Aβ40 in water and in 0.5 M ATP solution, respectively. The contacts (CHC–CHC, CHC–SHR, and SHR–SHR) found in neat water are highlighted. (E) The preferential interaction coefficient (Γ) of ATP with each different part of Aβ40 protein (NTR, CHC, TR, SHR, and CTR) is being shown. (F) Interaction of Aβ40 protein chain with ATP cosolute. ATP molecules are being shown in vdw representation. (G) The interacting ATP molecules crowd around the two Aβ40 protein chains are being shown. (H) and (I) show the consequence of Aβ40 dimer in neat water and in presence of ATP, respectively. The corresponding simulation snapshots are being shown for simulation starting with Aβ40 dimer in water and in 0.5 M ATP in 50 mM NaCl solution. (J) represents the probability distribution of distance between the two protein copies of the preformed Aβ40 dimer in absence (50 mM NaCl solution) and presence of ATP (0.5 M ATP in 50 mM NaCl solution).
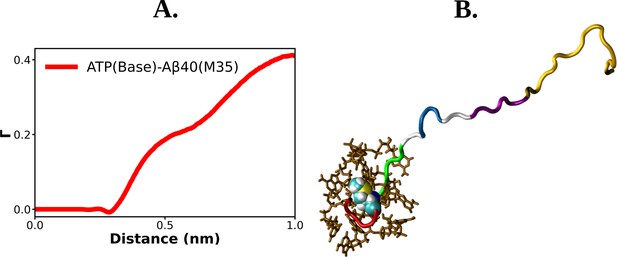
Residue-level details of ATP's interaction with Abeta40.
(A) shows the preferential interaction coefficient of adenosine triphosphate (ATP) with M35 residue of Aβ40. ATP molecules interacting with M35 residue of Aβ40 is shown with a snapshot in (B).
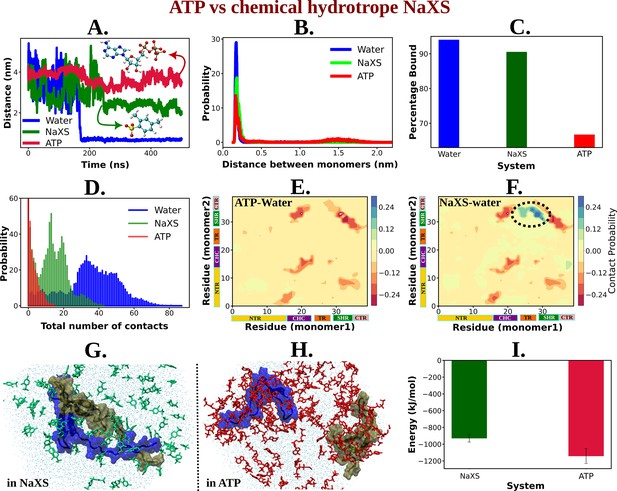
ATP's Efficiency as an Aggregate Solubilizer.
(A) shows the time profile of distance between the two protein chains (CHC–CHC) in neat water (blue curve), in 0.5 M NaXS (green curve) and adenosine triphosphate (ATP) (red curve) in 50 mM NaCl solution. (B) represents the probability distribution of the distance between the protein chains in each of the three (above mentioned) cases. (C) shows the percentage of bound of the proteins (for all the three systems) in a bar plot representation. (D) depicts the total number of intermolecular contacts of the protein monomers in each of the three solutions. (E) and (F) represent the difference of residue-wise inter-protein contact map of Aβ40 in 0.5 M ATP and 0.5 M NaXS solution, respectively, from that of the neat water system. (G) and (H) show the representative snapshots captured during the Aβ40 dimerization simulation in 0.5 M NaXS and 0.5 M ATP solution, respectively. Figure 1 represents the interaction energy (Coulombic interaction) between the NaXS (green bar) and ATP (red bar) molecules with the protein molecules in a bar plot representation. The vertical lines (black colored) show the error bars in the estimation.
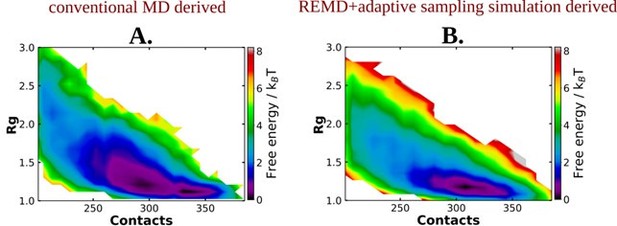
Image represents the 2D free energy profile for Aβ40 monomer in absence of ATP, obtained through A.
conventional MD and B. REMD simulation followed by adaptive sampling simulation.
The time profile of temperature (a, c, e and g) and energies i.
e. kinetic energy, potential energy and total energy (b, d, f and h) are being represented for Trp-cage in absence (a-b) and presence of 0.5 MATP (c-d) and Aβ40 protein in absence (e-f) and presence of 0.5 M ATP (g-h).







