Bridging verbal coordination and neural dynamics
Figures
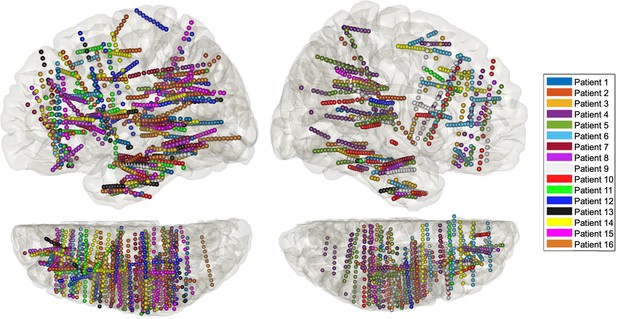
Anatomical localisation of the stereotactic EEG (sEEG) electrodes for each patient projected in MNI space on the lateral 3D view (top) and on the top view (N =16).
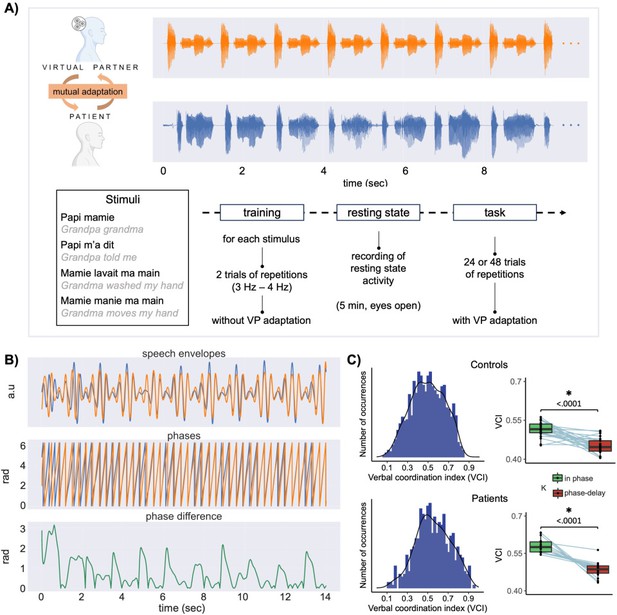
Paradigm and coordination indices.
(A) Top: illustration of one trial of the interactive synchronous speech repetition task (orange: virtual partner [VP] speech; blue: participant speech; stimulus papi m’a dit repeated 10 times; only the 10 first seconds are represented). Bottom: the four speech utterances used in the task and the experimental procedure. (B) Speech signals processing stages. The top panel corresponds to the speech envelope, the second to the phase of speech envelope and the third panel to the phase difference between VP and participant speech envelopes, illustrating the coordination dynamics along one trial. (C) Left: distributions of verbal coordination index (phase locking values between VP and participant speech envelopes, for each trial) for all participants (top) and patients. Right: boxplots for control participants (top) and patients showing the trial-averaged verbal coordination index as a function of the VP parameters (in-phase coupling vs coupling with a 180° shift).
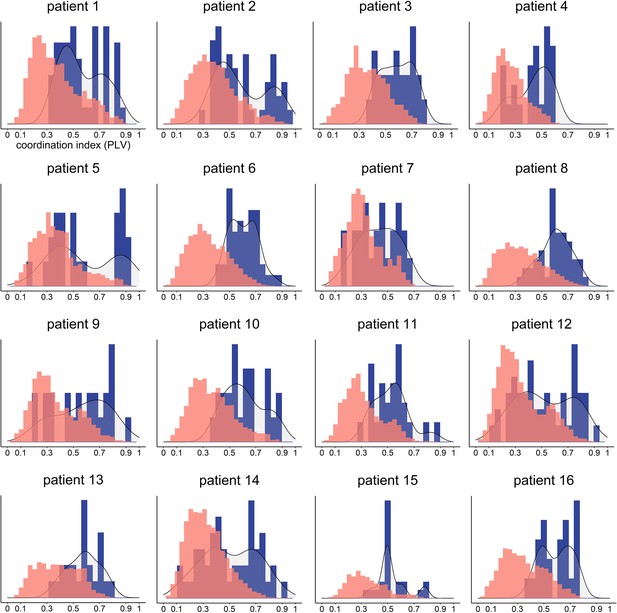
Distribution of verbal coordination index for each patient (PLV between patient’s speech and virtual partner [VP] speech).
For each of the 16 patients, this figure depicts the histogram of the coordination index for all trials (in blue) as well as the null distribution (random phase shift) computed using 500 permutations per trial (in red).
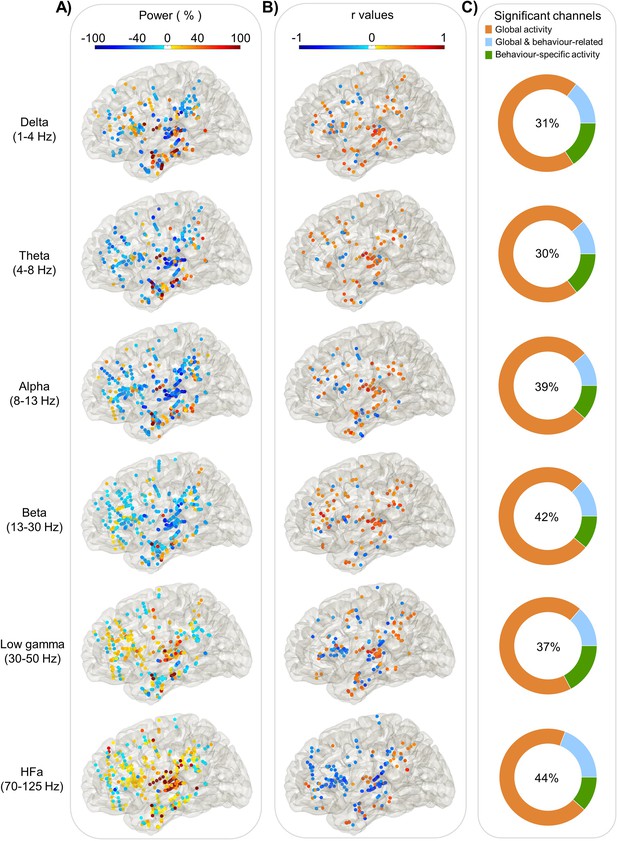
Power spectrum analyses and correlation with verbal coordination index (VCI; left hemisphere).
Each dot represents a channel where a significant effect was found either on (A) Global activity (Task vs Rest) for each frequency band. The activity is expressed in % of power change compared to resting; or on (B) Behaviour-related activity: r values of the Spearman correlation across trials between the iEEG power and the VCI. (C) The proportion of channels where a significant effect was found: in the task versus rest (orange), in the brain–behaviour correlation (green) or for both comparisons (blue). The percentage in the centre indicates the overall proportion of significant channels from the three categories with respect to the total number of channels.
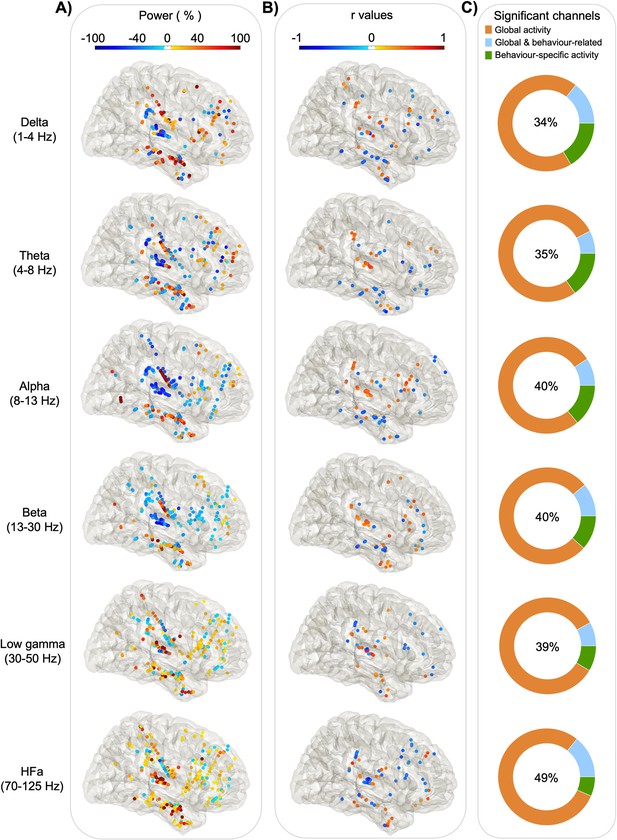
Power spectrum analyses and correlation with verbal coordination index (VCI; right hemisphere).
Each dot represents a channel where a significant effect was found either on (A) global activity (Task vs Rest) for each frequency band. The activity is expressed in % of power change compared to resting; or on (B) behaviour-related activity: r values of the Spearman correlation across trials between the iEEG power and the VCI. (C) The proportion of channels where a significant effect was found: in the task versus rest (orange), in the brain–behaviour correlation (green) or for both comparisons (blue). The percentage in the centre indicates the overall proportion of significant channels from the three categories with respect to the total number of channels.
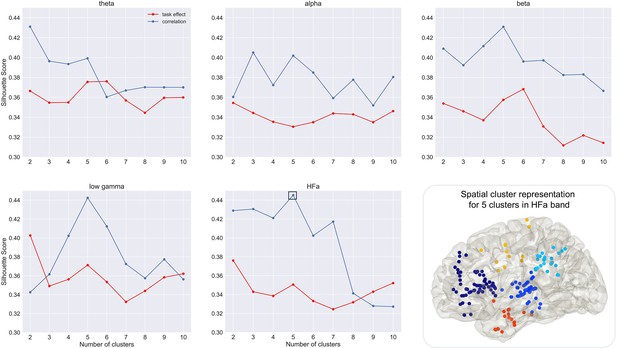
Cluster analysis (silhouette score).
Illustration of the mean silhouette scores according to the number of clusters for global activity (in red) and behaviour-related activity (correlation between power changes and coordination index, in blue). The highest silhouette score was obtained for five clusters in the high-frequency activity (HFa) range for behaviour-related activity (framed in full black square). Bottom right: spatial cluster representation for the highest mean silhouette score value in HFa range.
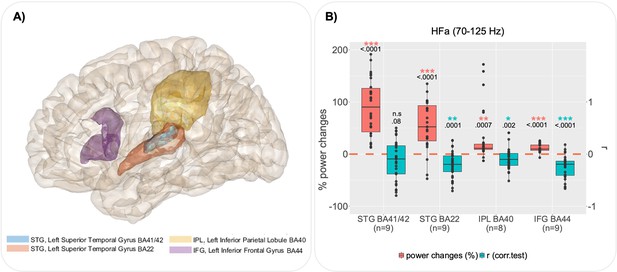
Group analysis by regions of interest (ROI) for the left hemisphere.
(A) ROI defined according to the cluster analysis (see Figure 3—figure supplement 2), the delimitation of regions is based on the Brainnetome atlas. (B) For each ROI, boxplots illustrate, in red, channels with significant global power changes (high-frequency activity [HFa], task vs rest) and, in blue, their corresponding r values (correlation between HFa power and verbal coordination index, VCI). Red and blue stars indicate a significant difference from a null distribution. Dots represent independent iEEG channels. The ‘n’ below each ROI specifies the number of patients. STG: superior temporal gyrus; IPL: inferior parietal lobule; IFG: inferior frontal gyrus; BA: Brodmann area.
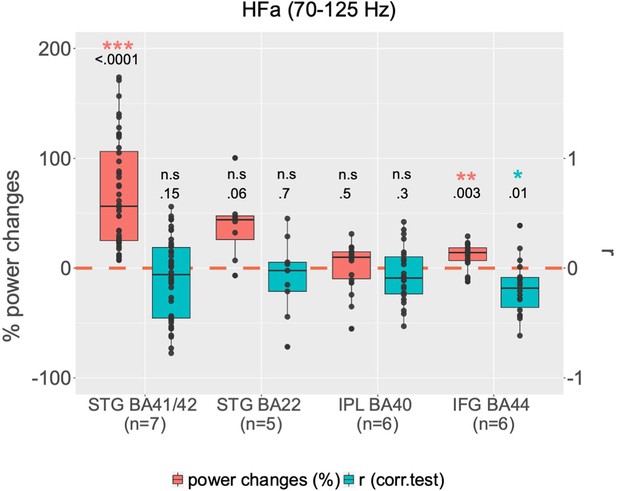
Group analysis by regions of interest (ROI; right hemisphere).
For each ROI, boxplots illustrate, in red, channels with significant global power changes (high-frequency activity [HFa], task vs rest) and, in blue, their corresponding r values (correlation between HFa power and verbal coordination index, VCI). Red and blue stars indicate a significant difference from a null distribution. Dots represent independent iEEG channels. The ‘n’ below each ROI specifies the number of patients. STG: superior temporal gyrus; IPL: inferior parietal lobule; IFG: inferior frontal gyrus; BA: Brodmann area.
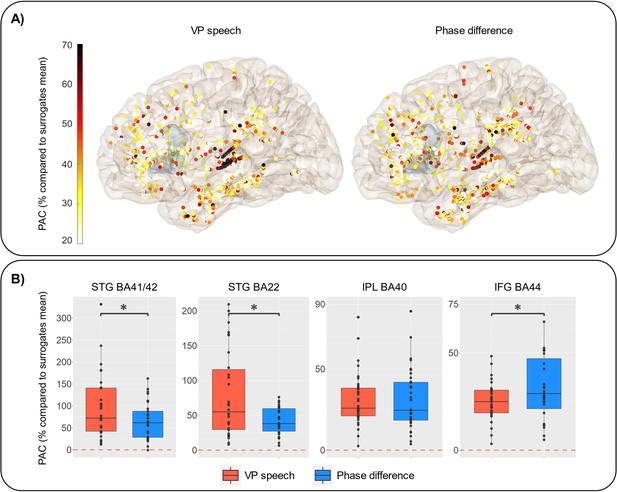
Phase-amplitude coupling (PAC) between virtual partner [VP] speech signal or coordination dynamics and high-frequency activity (HFa).
(A) Representation of the increase in PAC expressed in % compared to surrogates mean when using the VP speech (left) or the coordination dynamics (phase difference between VP and patient, right). The shaded (slight blue) area corresponds to the location of the inferior frontal gyrus (IFG) BA44. (B) PAC values for VP (in red) and phase difference (in blue) by regions of interest. Statistical difference between the two types of PAC is calculated using paired Wilcoxon’s test (STG BA41/42: p = 0.01; STG BA22: p = 0.004; inferior parietal lobule [IPL] BA40: p = 0.6; IFG BA44: p = 0.02). Y-axis range has been adjusted to better illustrate the contrast between VP speech and coordination dynamics.
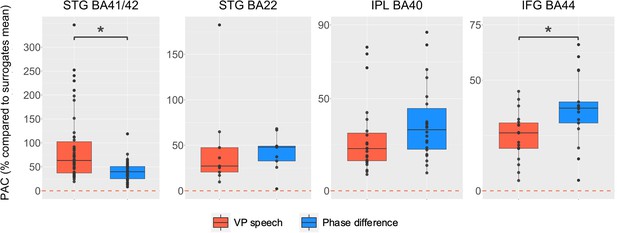
Phase-amplitude coupling (PAC) analyses by region of interest (right hemisphere).
PAC expressed in % compared to surrogates when using as phase the virtual partner [VP] speech (in red) or the coordination dynamic (phase difference between VP and participant, in blue) and as amplitude the high-frequency activity. Statistical difference between the two different types of PAC is calculated using paired Wilcoxon test (STG BA41/42: p < 0.0001; STG BA22: p = 0.9; inferior parietal lobule [IPL] BA40: p = 0.2; inferior frontal gyrus [IFG] BA44: p = 0.01). Y-axis range has been adjusted to better illustrate the contrast between VP speech and coordination dynamic.
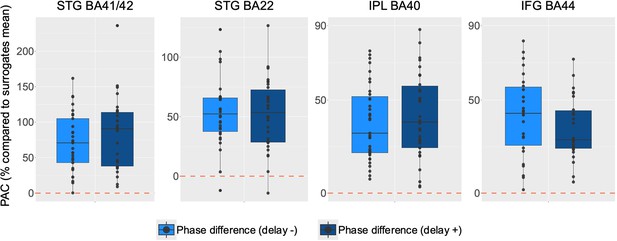
Phase-amplitude coupling (PAC) according to the behavioural delay (left hemisphere).
PAC expressed in % compared to surrogates when using as phase the coordination dynamic (phase difference between virtual partner [VP] and participant) and as amplitude the high-frequency activity. Comparison between PAC on trials with negative and positive delays (see ‘Coupling behavioural and neurophysiological data’ section in Materials and methods). Please note that the y-axis range has been adjusted per panel.





