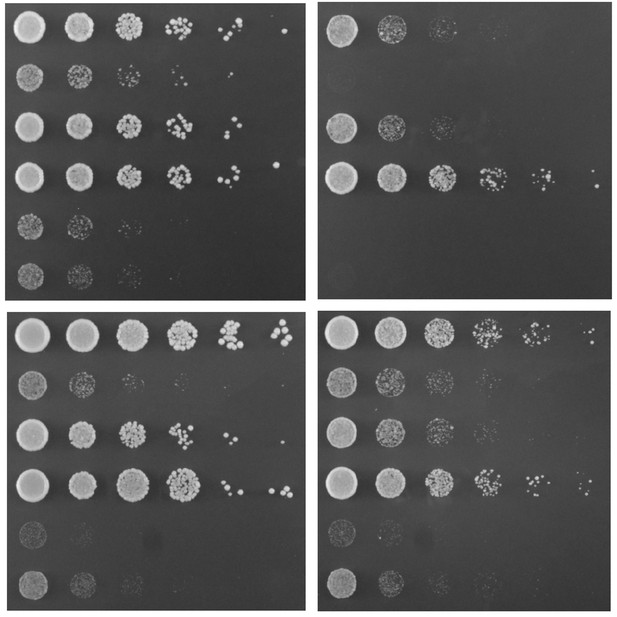
Mutations to enzymes that repair and decay RNA affect the unfolded protein response and alter how well yeast cells grow. Image credit: Cherry et al. (CC BY 4.0)
Like any economical factory, cells tune the size of their protein assembly line to suit demand. Proteins consist of strings of amino acids, built from template molecules called mRNAs, that must be folded into specific 3D structures for them to work correctly. If these protein strings are produced faster than they can be folded, the cell triggers the unfolded protein response. This response slows protein production, gets rid of any misshapen proteins, and increases the size of the protein assembly line.
It is not clear exactly how the unfolded protein response is tuned, though an mRNA molecule called HAC1 is known to signal the response. First, enzymes remove a short section of HAC1 and join the remaining parts back together in a process called splicing. Spliced HAC1 is then used as a template to make a protein that activates the unfolded protein response.
To understand more about this processing of HAC1, Cherry et al. studied yeast cells that had mutated, non-working versions of some of the enzymes that repair and degrade RNA. This revealed that the splicing of HAC1 competes with another process that breaks down mRNA. Under normal conditions, this means that HAC1 is degraded before it can trigger the unfolded protein response. In addition, for the cell to trigger the unfolded protein response, it needs to break down the part of HAC1 that is removed during splicing. Otherwise, the removed section interferes with the spliced HAC1 mRNA, preventing it from being a signal to activate the unfolded protein response.
Cherry et al. also found that a unique, chemically modified fragment of HAC1 mRNA was protected from degradation. They do not know how the unique chemical modification regulates the unfolded protein response, but stabilizing modifications are generally useful in RNA biology.
Understanding how the unfolded protein response is tuned could help researchers to find new ways to treat conditions where it does not work correctly, such as neurodegeneration, diabetes and cancer. Additionally, researchers are already trying to develop treatments for a number of diseases that work by inserting new RNA molecules into cells. Understanding how the chemical modification discovered by Cherry et al. protects RNAs from degradation could therefore improve the effectiveness of such treatments.




