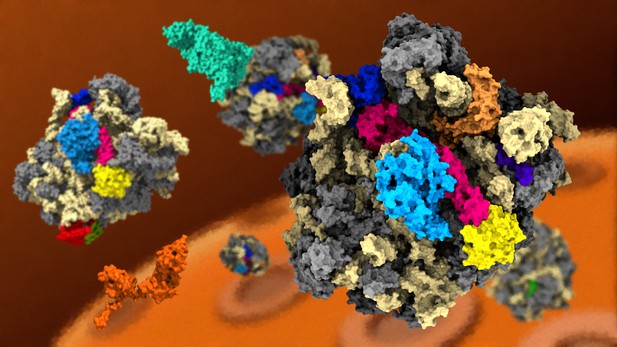
The final stages of ribosome assembly occur outside the cell nucleus. Image credit: Norberto Escudero-Urquijo (CC BY 4.0)
Biological machines called ribosomes make proteins in the cells of our body. Mammalian cells build roughly 7,500 new ribosomes every minute, each one containing 80 proteins and four RNA molecules. Problems that prevent ribosomes from assembling correctly have been linked to cancers such as leukemia, and a class of disorders called ribosomopathies that increase the likelihood of someone developing cancer. Understanding how ribosomes assemble could therefore help to develop new treatments for these diseases.
Ribosomes are mostly constructed in the cell nucleus, but the final stages of assembly occur in the cytoplasm of the cell. A protein called Nmd3 binds to the partly constructed ribosome to export it out of the nucleus. Then, the final ribosomal proteins integrate into the structure to form a key site called the peptidyltransferase centre (PTC), which is where the ribosome joins together amino acids when making new proteins for the cell. Questions remained about how these final assembly steps occur, and how Nmd3 is removed from the ribosome.
Kargas et al. have now examined how the PTC forms by using a method known as cryo-electron microscopy to determine the structures that the ribosome forms at different stages of assembly. This revealed that when the last two ribosomal proteins integrate into the ribosome, the ribosomal RNA goes through large shape changes that evict Nmd3 from the PTC. Quality control factors then check the structure of the newly formed ribosome and, if it passes their checks that it works correctly, license it to start making cell proteins.
This stage of ribosome assembly is likely to occur in the same way in all plant, animal and other eukaryotic species. The results presented by Kargas et al. will also help researchers to better understand the consequences of the mutations that affect ribosomal proteins in cancer cells. Ultimately, this knowledge may help to uncover new ways to treat cancer and ribosomopathies.




