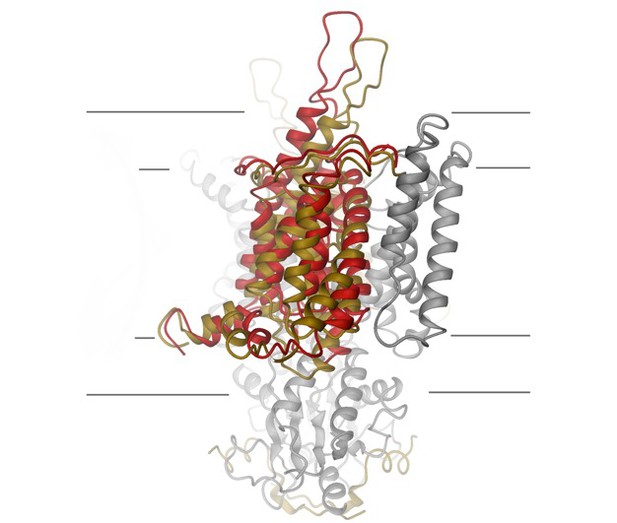
The structure of a protein internal to the membrane called SLCA9, which helps transport chloride ions in the lung. Image credit: Raimund Dutzler (CC BY 4.0)
Many processes in the human body are regulated by chloride and other charged particles (known as ions) moving in and out of cells. Each cell is surrounded by a membrane barrier, which prevents ions from entering or exiting. Therefore, to control the levels of ions inside the cell, specific proteins in the membrane act as channels or transporters to provide routes for the ions to pass through the membrane.
Channel proteins form pores that, when open, allow a steady stream of ions to pass through the membrane. Transporter proteins, on the other hand, generally contain a pocket that is only accessible from one side of the membrane. When individual ions enter this pocket the transporter changes shape. This causes the entrance of the pocket to close and then re-open on the other side of the membrane.
Inside the lung, an ion channel known as CFTR provides a route for chloride ions to move out of cells, which helps clear harmful material from the airways. Mutations affecting this protein cause the mucus lining the airways to become very sticky, leading to a severe disease known as cystic fibrosis. CFTR works together with another protein that is also found in the membrane, called SLC26A9. Previous studies have suggested that SLC26A9 also allows chloride ions to pass through the membrane. It was not clear, however, if SLC26A9 operates as an ion channel or a transporter protein, or how the protein is arranged in the membrane.
Now, Walter, Sawicka and Dutzler combined two techniques known as cryo-electron microscopy and patch-clamp electrophysiology to reveal the detailed three-dimensional structure of the mouse version of SLC26A9, which is highly similar to the human form. The experiments found that mouse SLC26A9 proteins form pairs in the membrane referred to as homodimers, which arranged themselves in an unexpected way. Further investigation into the structure of these homodimers suggests that despite having many channel-like properties, SLC26A9 operates as a fast transporter, rather than a true channel.
These findings help us understand the role of SLC26A9 and other similar proteins in the lung and other parts of the body. In the future it may be possible to develop drugs that target SLC26A9 to treat cystic fibrosis and other severe lung diseases.




