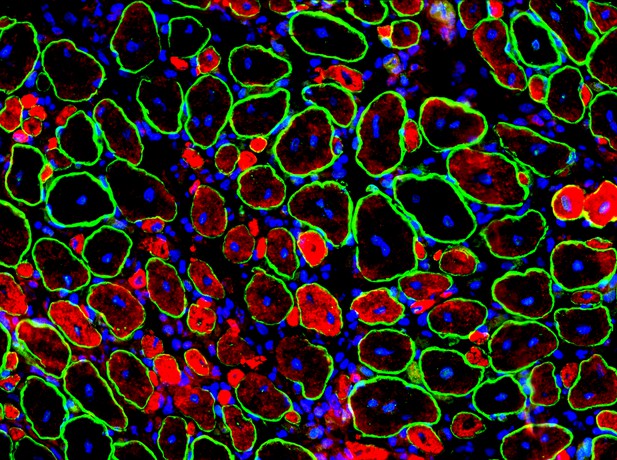
Muscle section one week after being injured: The membrane of the muscle cells is shown in green, nascent muscle cells are shown in red and the DNA is shown in blue. Image credit: Zhihao Jia (CC BY 4.0)
Muscles have their own population of stem cells, called muscle satellite cells. These cells are essential for muscle growth and repair. In healthy adult muscles, they spend most of their time inactive, but when there is an injury, they reawaken and start dividing. Some of the new cells return to an inactive stem cell state to await the next injury. The rest mature into new muscle cells or join with damaged muscle fibres to help them repair.
The cell cycle is the series of events that a cell goes through from its birth until it divides. In muscle satellite cells, progression through the cell cycle is tightly controlled to ensure they divide and grow the correct amount. One of the proteins responsible for controlling the cell cycle is Polo-Like Kinase 1 (PLK1), but studying this protein is difficult. A common way to investigate a protein's effect is to delete the gene that makes it and observe the consequences. However, PLK1 is so essential to life that yeast, flies, zebrafish and mice all die when the gene is missing. Jia et al. deleted the gene that makes PLK1 only in mouse muscle satellite cells to find out the role this protein plays in controlling the cell cycle in stem cells.
Deleting the gene that codes for PLK1 before the mice were born was lethal. The embryos failed to develop mature muscle fibres, and they died. But deleting the gene after the mice were born had a different effect. The muscles developed normally, but they were unable to heal when injured. The same healing problem also happened when healthy mice received a drug that blocked the function of PLK1 protein. A closer look at the muscle satellite cells revealed the source of the problem. Without PLK1, the cells got stuck part way through their cell cycle, just before they were due to divide. They tried to become muscle cells, but they did not make it. Instead, the muscle satellite cells started to act as though their DNA had been damaged, and then they self-destructed.
Muscle satellite cells become less able to divide as we get older. They can also malfunction in some types of degenerative muscle diseases. Understanding how muscle satellite cells control their cell cycle could help us to find out what causes them to go wrong. Further work to understand PLK1 also has potential implications for cancer treatment. PLK1 blockers have been used to stop cancer cells from dividing, but Jia et al.’s findings show that this kind of drug may also hamper the ability of muscle to repair damage.




