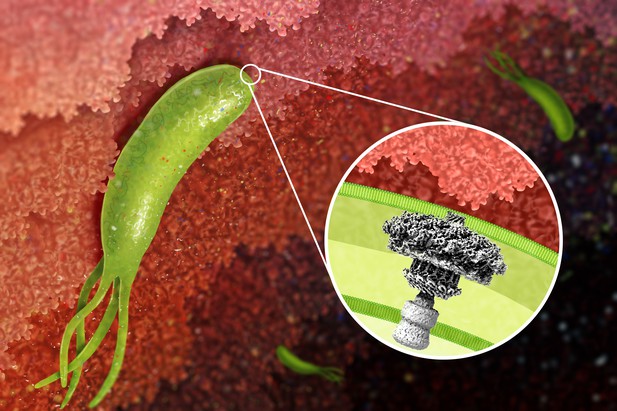
Artistic rendering of Helicobacter pylori attached to the stomach lining. The type IV secretion system (inset) transfers the bacterial protein CagA into cells of the human stomach. Jeong Min Chung and Stephanie King (CC BY 4.0)
Helicobacter pylori is a species of bacterium that can colonize the human stomach, causing changes that greatly increase the risk of ulcers and stomach cancer. Some strains of H. pylori produce a protein called CagA, which alters how stomach cells grow and divide. The bacterium injects CagA directly into stomach cells using a syringe-like structure called a type IV secretion system.
Type IV secretion systems are found in many species of bacteria and are involved in a variety of processes, including the exchange of genes between neighboring bacteria. The systems typically have at least 12 components. Previous studies have revealed how the components of some of these systems fit together to form working machines. However, the type IV secretion system that delivers CagA (called the Cag T4SS) contains additional components and it remains unclear how these components are organized in the structure.
A technique called cryo-electron microscopy uses electrons to visualize proteins that have been rapidly frozen so they can be captured and imaged in their natural shape and form. Chung, Sheedlo et al. extracted the Cag T4SS apparatus directly from H. pylori and used cryo-electron microscopy to determine its shape to a high level of detail. These images were then used to build a detailed model of the Cag T4SS that included many of its components.
The model shows that the Cag T4SS is larger and more complex than other type IV secretion systems that have been studied previously. Therefore, Chung, Sheedlo et al. propose that the Cag T4SS is specially adapted to work in the stomach.
These findings open the door for future research to define how individual components of the Cag T4SS help to inject CagA into stomach cells. In addition, future research will allow researchers to understand how the type IV secretion systems found in different bacterial species carry out a wide range of roles.




