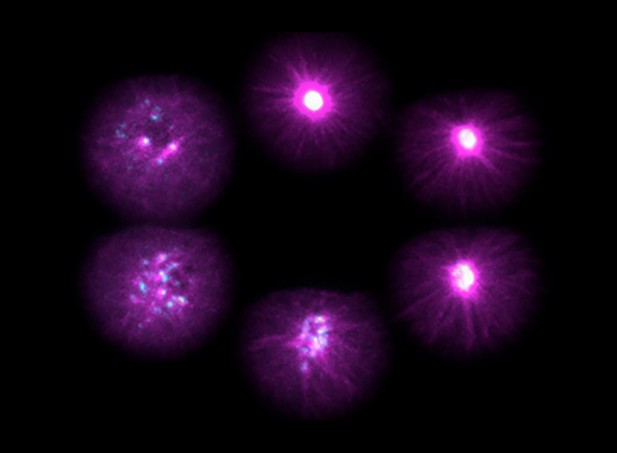
The phases of microtubule (magenta) and PCM (blue) disassembly from the centrosome. Image credit: Jeremy Magescas (CC BY 4.0)
New cells are created when existing cells divide, a process that is critical for life. A structure called the spindle is an important part of cell division, helping to orient the division and separate parts of the old cell into the newly generated ones. The spindle is built using filamentous protein structures called microtubules which are arranged by microtubule organizing centers (or MTOCs for short). In animals, an MTOC forms at each end of the spindle around two structures called centrosomes.
A network of proteins called the pericentriolar material (PCM) form around centrosomes, converting them into MTOCs. The PCM grows around centrosomes as a cell prepares to divide and is removed again afterward. Enzymes called kinases are important in controlling cell division and PCM assembly; they are opposed by other enzymes known as phosphatases. The processes involved in organization and removal of the PCM are not well understood.
The microscopic worm Caenorhabditis elegans provides an opportunity to study details of cell division in a living animal. Magescas et al. used fluorescent labels to view proteins from the PCM under a microscope. The images showed two partially overlapping spherical parts to the PCM – inner and outer. Further examination revealed that the inner PCM is maintained by a careful balance of kinase and phosphatase activity. When kinases shut down at the end of cell division, the phosphatases break down the inner PCM. By contrast, the outer PCM is physically torn apart by forces acting through the attached microtubules.
Future work will seek to examine which proteins are specifically affected by phosphatases to identify the key regulators of PCM persistence in the cell and to reveal the proteins needed for MTOC activity at the centrosome. Since poor MTOC regulation can play a part in the growth and spread of cancer, this could lead to targets for new treatments.




