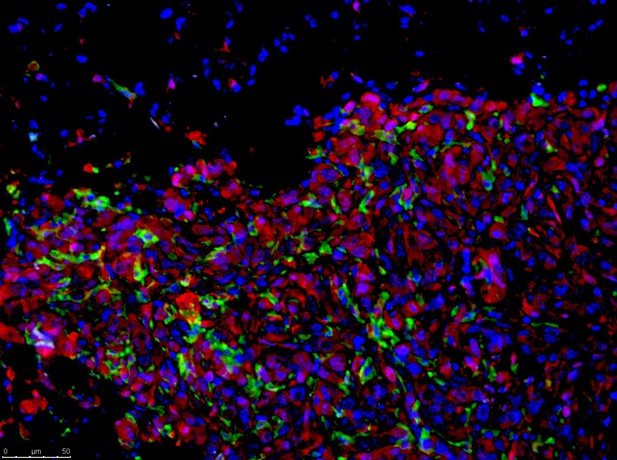
Liver cancer cells with high levels of MYC (red) and TWIST1 spreading into a lung that is infiltrated with macrophages (green) that have been converted to a tumor-friendly state. Image credit: Nia Adeniji (CC BY 4.0)
Cancer develops when cells in the body gain mutations that allow them to grow and divide rapidly and uncontrollably. As the disease progresses these cancer cells develop the ability to spread around the body. This process of spreading, called metastasis, is responsible for most cancer-related deaths in humans, but no current treatments target it.
Mutations that increase the levels of two proteins known as MYC and TWIST1 in cells cause many human cancers. In healthy adult cells, normal levels of MYC and TWIST1 act as key regulators that switch thousands of genes on or off. TWIST1 is known to control the movement and spread of cells in the embryo. However, it is not known how MYC and TWIST1 work together to promote the metastasis of cancer cells.
To address this question, Dhanasekaran, Baylot et al. used mice to investigate the roles of MYC and TWIST1 in the metastasis of cancer cells. The experiments showed that these two proteins work together to reprogram mouse cancer cells to release signal molecules known as cytokines. These molecules convert immune cells known as macrophages to a tumor-friendly state that allows cancers cells to spread around the body. Inhibiting two cytokines known as CCL2 and IL13 prevented the cancer cells from moving.
Further experiments analyzed tumor samples from around 10,000 human patients with 33 different cancers. This revealed that patients that had higher levels of MYC and TWIST1 proteins in their tumors also had increased levels of CCL2 and IL13, more activated macrophages and were less likely to recover from their cancer.
The findings of Dhanasekaran, Baylot et al. suggest that MYC and TWIST1 may instigate metastasis in many human cancers, and therapies targeting specific cytokines may prevent these cancers from spreading around the body. Furthermore, screening blood for the levels of cytokines may help to identify the cancer patients who would benefit from such therapies.




