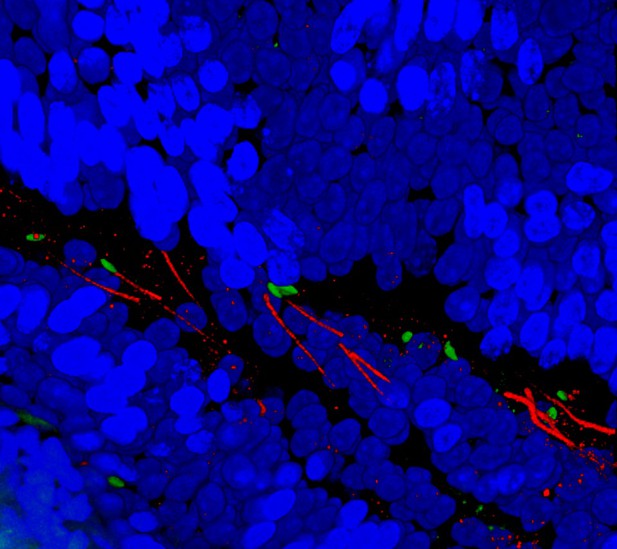
Three-dimensional image showing the head (green) and tail (red) of sperm cells travelling towards the fertilization site (to the left side of the image) in the reproductive tract (blue cells) of a female mouse. Image credit: Lukas Ded (CC BY 4.0)
When mammals mate, males ejaculate millions of sperm cells into the females’ reproductive tract. But as the sperm travel up the tract, only a handful of the ‘fittest’ sperm will actually manage to reach the egg. This process of elimination prevents the egg from being fertilized by multiple sperm cells and stops the eggs from being fertilized outside of the womb. A lot of what is known about fertilization in mammals has come from studying how sperm and eggs cells interact in a Petri dish. However, this approach cannot explain how sperm are selected and removed as they journey towards the egg.
Previous work suggests that a calcium channel, which sits in the membrane surrounding the sperm tail, may provide some answers. The core of this channel, known as CatSper, is made up of four proteins arranged into a unique pattern similar to racing stripes. Without this specific arrangement, sperm cells cannot move forward and fertilize the egg in time. To investigate the role of this protein in more depth, Ded et al. established a new way to image the minute structures of sperm cells, such as CatSper, in the reproductive tract of female mice.
Experiments in a Petri dish revealed that sperm cells that have been primed to fertilize the egg are a diverse population: in some cells one of the proteins that make up the calcium channel, known as CatSper1, is cleaved, while in other cells this protein remains intact. Visualizing this protein in the female reproductive tract showed that sperm cells close to the site of fertilization contain non-cleaved CatSper1. Whereas sperm cells further away from the egg – and thus closer to the uterus – are more likely to contain broken down CatSper1.
Taken together, these findings suggest that the state of the CatSper1 protein may be used to select sperm that are most likely to reach and fertilize the egg. Future studies should address what happens to the calcium channel once the CatSper1 protein is cleaved, and how this channel controls the movements and lifespan of sperm. This could help identify new targets for contraception and improve current strategies for assisted reproduction.




