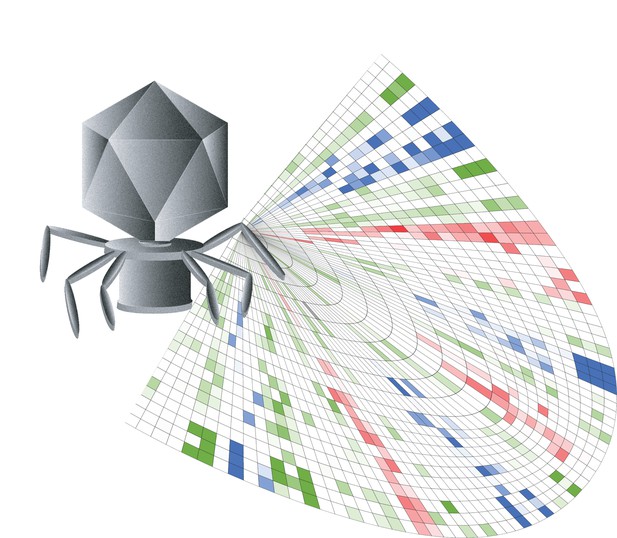
Bacteria-killing viruses (grey) recognize their prey through a structure called RBP; mapping out the role of each of RBP sections (colorful squares) helps to design viruses that can prey on specific strains of bacteria. Image credit: Phil Huss (CC BY 4.0)
Bacteria can cause diseases, but they also battle their own microscopic enemies: a group of viruses known as bacteriophages. For instance, the T7 bacteriophage preys on various strains of Escherichia coli, a type of bacteria often found in the human gut. While many E. coli strains are inoffensive or even beneficial to human health, some can be deadly. Finding a way to kill harmful strains while sparing the helpful ones would be a helpful addition to the medicine toolkit.
Bacteriophages identify and interact with their specific target through a structure known as the receptor binding protein, or RBP. However, it is still unclear exactly how RBP helps the viruses recognize which type of bacteria to infect. Here, Huss et al. set to map out and modify this structure in T7 bacteriophage so the virus is more efficient and specific about which strain of E. coli it kills.
First, the role of each building block in the tip of RBP was meticulously dissected; this generated the knowledge required to genetically engineer a large number of different T7 bacteriophages, each with a slightly variation in their RBP. These viruses were then exposed to various strains of bacteria. Monitoring the bacteriophages that survived and multiplied the most after infecting different strains of E. coli revealed which RBP building blocks are important for efficiency and specificity. This was then confirmed by engineering highly active T7 bacteriophage variants against an E. coli strain that causes urinary tract infections.
These findings demonstrate that even small changes to the bacteriophages can make a big difference to their ability to infect their preys. The approaches developed by Huss et al. help to understand exactly how the RBP allows a virus to infect a specific type of bacteria; this could one day pave the way for new therapies that harness those viruses to fight increasingly resistant bacterial infections.




