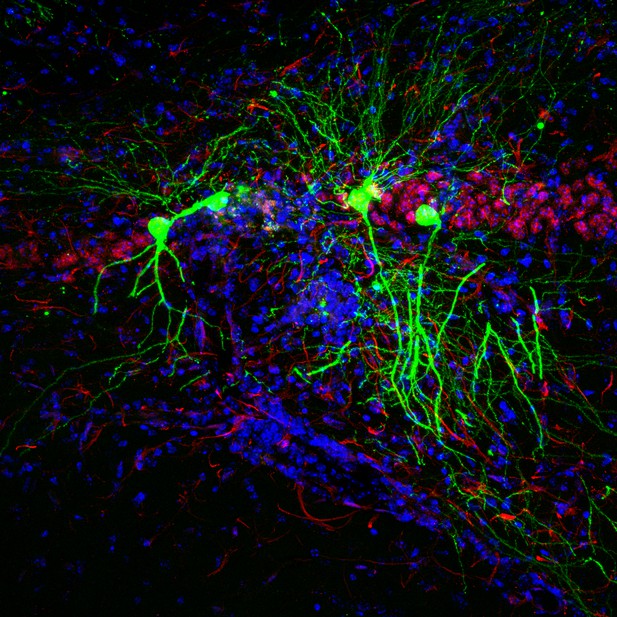
Hippocampal neurons of mice injected with the Toxoplasma protein (green). Other neurons and cell nuclei shown in red and blue, respectively. Image credit: Mendez et al. (CC BY 4.0)
Toxoplasma gondii is an intracellular parasite that infects the brain. Whereas most microbial infections of the brain result in severe illness or death, Toxoplasma gondii infections are usually asymptomatic. This is because the parasite has evolved the ability to exist within the brain by dampening the immune response. The parasite can therefore asymptomatically co-exist with its host for years – or even an entire lifetime. The strategy has proved so successful that up to one third of the world’s population is now thought to be infected with Toxoplasma gondii.
While this persistence tends not to be a problem for most healthy individuals, dormant Toxoplasma gondii parasites can reactivate in individuals whose immune systems fail. This can result in life-threatening neurological disease. In pregnant women, Toxoplasma gondii parasites can also cross the placenta, which can trigger miscarriage or cause harmful disease in the newborn.
To develop treatments for these cases of symptomatic disease, we need to understand how the parasite hides from the immune system in asymptomatic individuals. Mendez et al. have therefore leveraged a mouse model in which neurons injected with Toxoplasma gondii proteins (Toxoplasma-injected neurons, or ‘TINs’) produce a green fluorescent protein. This enables the infected cells to be viewed under a microscope.
Examining the mouse brains revealed that most TINs were located in two specific regions: the cortex and the striatum. The cortex is the brain’s outer layer of tissue. The striatum is a structure deep within the brain that helps regulate movement and responses to rewards. Both the cortex and the striatum contain different types of neurons. The results revealed that the proteins from the parasite were spread roughly equally among the various cell types, rather than targeting a specific subtype of neuron.
Neurons close to TINs had slightly abnormal electrical activity, whereas the TINs themselves had highly abnormal activity. By eight weeks post-infection, however, the number of TINS had fallen by around 90%. This suggests that many neurons containing Toxoplasma protein are sick and dying, and that their altered electrical activity reflects this unhealthy state.
Understanding how Toxoplasma parasites persist in the brain has the potential to reveal new targets for treating symptomatic infections. It could even provide new possibilities for targeting the inflammation that drives many other neurological diseases. Harnessing this potential will require finding out why Toxoplasma gondii infects specific brain regions and why most neurons that directly interact with the parasite die.




