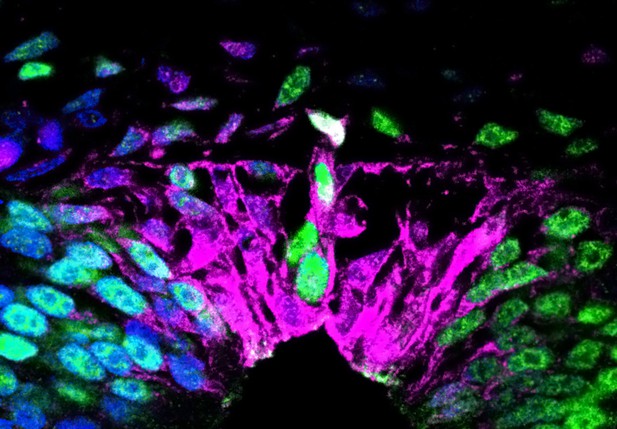
In the absence of retinoic acid, the nascent roof plate contains cells that produce both proteins normally only found in the roof plate, such as Rspo1 (shown in magenta) as well as proteins that are neural-crest specific, such as Sox9 (shown in green). Image credit: Dina Rekler (CC BY 4.0)
The division between the central nervous system – formed by the brain and spinal cord – and the peripheral nervous system – which consists of the neurons that sense and relay information to and from the body – takes place early during embryonic development. Initially, the nervous system consists of a tube of cells called the neural tube. From the top region of this tube, some cells change their shape, exit the tube and migrate to different places in the developing body. These cells are called the ‘neural crest’, and they form many different structures, including the peripheral nervous system.
Neural crest cells keep leaving the neural tube for a period of time, but after that, the neural tube stops producing them. At this point, the region of the neural tube that had been producing neural crest cells becomes the ‘roof plate’ of the central nervous system, a structure that is essential for the development of specific groups of neurons in the brain and spinal cord.
In bird embryos, a protein called bone morphogenetic protein (BMP) is essential for neural crest production because it triggers the migration of these cells away from the neural tube. Before the roof plate is formed, the activity of BMP is blocked by proteins known as BMP inhibitors, which stop more cells from leaving the neural tube. Around the time when neural crest formation stops, another molecule called retinoic acid begins to be synthesized in the top region of the neural tube. Rekler and Kalcheim asked whether retinoic acid is involved in the transition from neural crest to roof plate.
To test this hypothesis, Rekler and Kalcheim blocked the activity of retinoic acid in the neural tube of quail embryos at the time when they should stop producing neural crest cells. This resulted in embryos in which the neural tube keeps producing neural crest cells after the roof plate has formed. In these embryos, individual cells in the resulting ‘roof plate’ produced both proteins that are normally only found in neural crest cells, and proteins typically exclusive to the roof plate. This suggests that, in the absence of retinoic acid activity, the segregation of neural crest identity from roof plate identity is compromised.
Rekler and Kalcheim also found that, in the embryos where retinoic acid activity had been blocked, the cells in the area where the roof plate should be produced virtually no BMP inhibitors, and exhibited extended BMP activity. This allowed neural crest cells to continue forming and migrating away from the neural tube well after the period when they would stop in a normal embryo. These results indicate that retinoic acid stops the production of neural crest cells by repressing BMP activity in the roof plate of the neural tube.
Rekler and Kalcheim’s experiments shed light on the mechanisms that allow the central and peripheral nervous systems to become segregated. This could increase our understanding of the origin of several neurodevelopmental disorders, potentially providing insights into their treatment or prevention. Additionally, the process of neural crest production and exit from the neural tube is highly similar to the process of metastasis in many invasive cancers. Thus, by understanding how the production of neural crest cells is terminated, it may be possible to learn how to prevent malignant cancer cells from spreading through the body.




