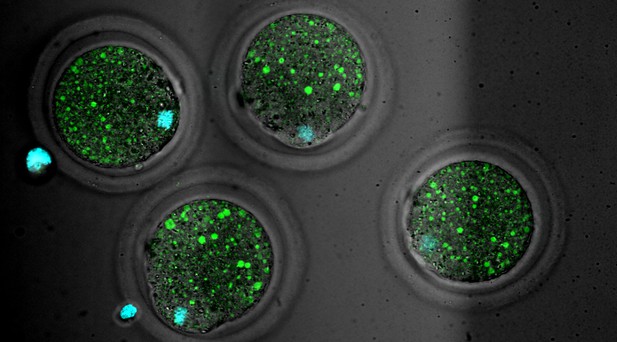
Mouse oocytes with the protein LAMP1 stained in green to show lysosomes and DNA stained in cyan. Image credit: Yuhkoh Satouh (CC BY 4.0).
Mammalian egg cells, known as oocytes, store the nutrients and materials required for early embryo development. Before fertilisation, they exist in a low-energy, almost hibernation-like state. However, once fertilised, oocytes break down their stored nutrients to support embryo growth.
This process partly relies on the digestive activity of organelles called lysosomes, which contain proteins that can break down cell contents. Nutrients and unnecessary cell contents are delivered to lysosomes via compartments known as endosomes and autophagosomes, where they are then recycled to be used in development. However, it is important that these compartments become active at precisely the right time in development. If they are activated too soon, the stored nutrients will be depleted. On the other hand, if they are activated too late, the embryo will not have the nutrients it needs to develop.
To investigate how the oocyte controls activation of endosome and lysosome activity, Satouh et al. used live imaging techniques to observe mice oocytes as they matured, were fertilised and developed into early embryos. This revealed that early in oocyte development, a large spherical structure forms, which grows as the egg matures.
Naming the structure an Endosomal-Lysosomal Organellar Assembly (or ELYSA for short), Satouh et al. showed that before fertilisation, endosomes and lysosomes are grouped within ELYSAs, which keeps them mostly inactive. After fertilisation, the ELYSAs break apart, with normal endosomal and lysosomal activity beginning at the same time.
These findings shed light on an important, previously unknown regulatory process in oocyte maturation and early embryonic development. Recently, it has been suggested that endosomal activity may be involved in determining the developmental potential of an embryo. Therefore, studying assembly and disassembly of ELYSAs in ageing oocytes may provide insights into how fertility changes, with the potential to help improve fertility assessments and treatments in the future.




