| Peer-reviewed | Experminental study | People/animals |
Scientists have pinpointed a possible new target for treating patients with the blood cancer juvenile myelomonocytic leukaemia (JMML), according to a study published today in eLife.
Their findings in zebrafish and JMML patients suggest that treatment using anti-inflammatories could be a possible new approach to combating the disease.
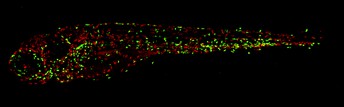
This image shows the macrophages (red) and neutrophils (green) in a zebrafish embryo with a mutation in SHP2. The head of the embryo is on the left, the tail on the right. Similar to the situation in JMML patients, this fish has more macrophages and neutrophils compared to fish without a mutation in SHP2. Image credit: Maja Solman (CC BY 4.0)
JMML is a highly aggressive blood cancer with poor outcomes for patients. Children with a relatively common developmental syndrome called Noonan Syndrome (NS) have a high risk of developing a condition similar to JMML, called myeloproliferative neoplasm, which can then progress to JMML. The most frequent genetic cause of JMML and NS is a mutation in the PTPN11 gene, which encodes the protein-tyrosine phosphatase SHP2.
“Hematopoietic stem and progenitor cells are considered to be the cells of origin for JMML,” says first author Maja Solman, Postdoctoral Fellow at the Hubrecht Institute, Utrecht, Netherlands. “Currently, hematopoietic stem cell transplantation is the only treatment for the disease, but it has a relapse rate of 50%. With such limited treatment options for JMML, we wanted to gain a better understanding of how the disease develops to identify other possible ways of targeting it.”
To do this, Solman and the team used a novel zebrafish model with a mutation in SHP2 – equivalent to the most common mutation in NS patients which can cause JMML. They used a technique called single-cell transcriptomics to examine the level of gene expression in the animals’ hematopoietic stem and progenitor cells. The analysis showed an increase in the number of monocyte and macrophage progenitor cells in the fish embryos, and that these cells expressed genes associated with the immune response.
The team next compared these results with their analysis of hematopoietic stem and progenitor cells, which contained SHP2 mutations, from the bone marrow of JMML patients. They found a similar pattern of proinflammatory gene expression in these cells as the one they identified in the zebrafish.
Finally, they treated the zebrafish embryos with an anti-inflammatory drug called dexamethasone. They found that the drug helped rescue JMML-like blood defects in the fish, suggesting that anti-inflammatories could one day be an important treatment strategy for JMML.
“Our work reveals striking similarities in the proinflammatory response of human and zebrafish cells containing SHP2 mutations, and shows that inhibiting this response can improve JMML-like symptoms in a zebrafish model,” concludes senior author Jeroen den Hertog, Group Leader and Managing Director at the Hubrecht Institute, and Professor of Molecular Developmental Zoology at Leiden University, Netherlands. “Together, these findings lay the groundwork for future studies to verify the effectiveness of anti-inflammatories as a potential new treatment approach for JMML patients.”
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373Melanie Fremery
Hubrecht Institute
m.fremery@hubrecht.eu
+31683596548
About
About eLife
eLife transforms research communication to create a future where a diverse, global community of scientists and researchers produces open and trusted results for the benefit of all. Independent, not-for-profit and supported by funders, we improve the way science is practised and shared. From the research we publish, to the tools we build, to the people we work with, we’ve earned a reputation for quality, integrity and the flexibility to bring about real change. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Cancer Biology research published in eLife, visit https://elifesciences.org/subjects/cancer-biology.
And for the latest in Developmental Biology, see https://elifesciences.org/subjects/developmental-biology.
About the Hubrecht Institute
The Hubrecht Institute is a research institute focused on developmental and stem cell biology. It encompasses 19 research groups that perform fundamental and multidisciplinary research, both in healthy systems and disease models. The Hubrecht Institute is a research institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), situated on Utrecht Science Park. Since 2008, the institute has been affiliated with the UMC Utrecht, advancing the translation of research to the clinic. The Hubrecht Institute has a partnership with the European Molecular Biology Laboratory (EMBL). For more information, visit http://www.hubrecht.eu.




