Researchers have identified how the bacterium that causes tuberculosis (TB) can evolve rapidly in response to new environments, says a study published today in eLife.
As with other species of bacteria, Mycobacterium tuberculosis (M. tuberculosis) is able to form complex structures called biofilms which allow bacterial cells to resist stressors such as antibiotics and immune cells. For this study, the research team evolved populations of M. tuberculosis in the lab and found that it could form thick biofilms due to mutations in genetic regions that cause multiple changes to happen at once. These findings could inform the development of antibiotics targeted at biofilm growth.
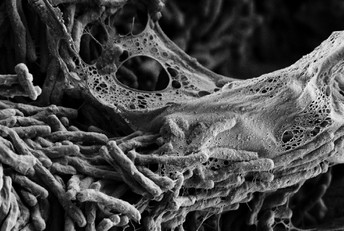
Mycobacterium tuberculosis cells within a biofilm. Image credit: John Kernien (CC BY 4.0)
As the second leading cause of death due to infectious disease globally, TB is a major threat to public health and there is an urgent need for new strategies for diagnosing, treating and controlling the infection.
“TB remains a challenging infection to treat due to the bacterium’s ability to persist in the face of antibiotic and immune pressure, and to acquire novel drug resistances,” explains Madison Youngblom, Graduate Student at senior author Caitlin Pepperell’s lab, University of Wisconsin-Madison, US, and co-first author of the study alongside Tracy Smith, New York Genome Center, New York City, US. “To better treat and control TB, we need to understand the sources of the bacterium’s robustness and identify its vulnerabilities. We wanted to learn more about how it is able to form biofilms by discovering the genes and genetic regions involved in biofilm growth, as well as how the bacterium evolves in response to changes in its environment.”
To do this, the team used experimental evolution of M. tuberculosis – a powerful tool for illuminating the strengths and vulnerabilities of the bacterium that has led to important insights on the fundamental processes that guide its adaptation. They evolved six closely related M. tuberculosis strains under selective pressure to grow as a biofilm. At regular intervals, they photographed the biofilm and described its growth according to four criteria: how much liquid surface the biofilm covered, its attachment to and growth up the sides of the dish, how thick the biofilm grew and the continuity of growth (compared to discontinuous patches of growth).
Their work revealed that each strain was able to adapt rapidly to environmental pressure, with the growth of a thicker and therefore more robust biofilm. The genetic regions that mutated during the experiment, causing this biofilm growth, were mostly regulators such as regX3, phoP, embR and Rv2488c. “These regulators control the activity of multiple genes, meaning a single mutation can cause many changes to occur in one go,” Youngblom explains. “This is an efficient process that we observed when we looked at the different characteristics of the bacteria, such as their cell size and growth rate.”
Additionally, the team found evidence suggesting that the genetic background of the parent strain of M. tuberculosis had an impact on the enhanced growth of the biofilms. This means that interactions between genetic factors could play an important role in the adaptation of the M. tuberculosis to changing environments.
“Bacteria are prone to growing as biofilms in many contexts, including the infection of humans and other hosts, and during colonisation of natural and built environments,” says senior author Caitlin Pepperell, Principal Investigator at the University of Wisconsin-Madison. “In a medical context, the insights gained from our work could be used to explore potential new antibiotics that are better able to attack bacteria that grow this way. We imagine such biofilm-directed therapies for TB would likely be add-ons to conventional therapy to help shorten and simplify current treatment strategies.”
Media contacts
Emily Packer
eLife
e.packer@elifesciences.org
+441223855373George Litchfield
eLife
g.litchfield@elifesciences.orgAndrea Schmick
University of Wisconsin Department of Medicine
aschmick@medicine.wisc.eduAndrew Hellpap
UW Health
AHellpap@uwhealth.org
About
eLife transforms research communication to create a future where a diverse, global community of scientists and researchers produces open and trusted results for the benefit of all. Independent, not-for-profit and supported by funders, we improve the way science is practised and shared. From the research we publish, to the tools we build, to the people we work with, we’ve earned a reputation for quality, integrity and the flexibility to bring about real change. eLife receives financial support and strategic guidance from the Howard Hughes Medical Institute, Knut and Alice Wallenberg Foundation, the Max Planck Society and Wellcome. Learn more at https://elifesciences.org/about.
To read the latest Genetics and Genomics research published in eLife, visit https://elifesciences.org/subjects/genetics-genomics.
And for the latest in Microbiology and Infectious Disease, see https://elifesciences.org/subjects/microbiology-infectious-disease.




