Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity
Figures
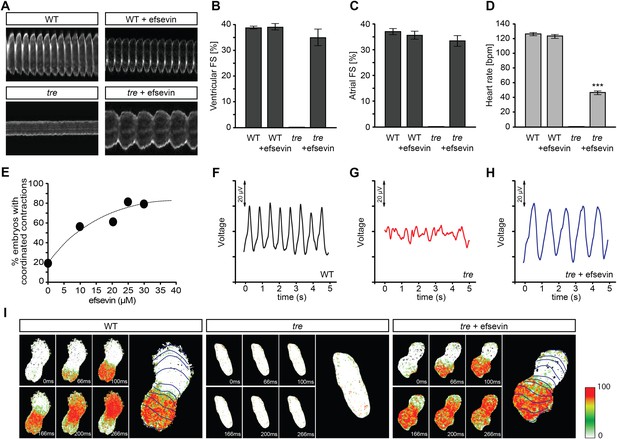
Efsevin restores rhythmic cardiac contractions in zebrafish tremblor embryos.
(A) Line scans across the atria of Tg(myl7:GFP) embryonic hearts at 48 hpf. Rhythmically alternating systoles and diastoles are recorded from vehicle- (upper left) or efsevin- treated wild type (upper right) and efsevin-treated tre (lower right) embryos, while only sporadic unsynchronized contractions are recorded from vehicle-treated tre embryos (lower left). (B, C) Fractional shortening (FS) deduced from the line-scan traces. While cardiac contraction was not observed in tre, efsevin-treated wild type and tre hearts have similar levels of FS to those observed in control hearts. Ventricular FS of wild type v.s. wild type + efsevin vs tre + efsevin: 39 ± 0.6%, n = 8 vs 39 ± 1%, n = 10 vs 35 ± 3%, n = 6; and Atrial FS: 37 ± 1%, n = 11 vs 35 ± 2%, n = 11 vs 33 ± 2%, n = 15. (D) While efsevin restored a heart rate of 46 ± 2 beats per minute (bpm) in tre embryos, same treatment does not affect the heart rate in wild type embryos (126 ± 2 bpm in vehicle-treated embryos vs 123 ± 3 bpm in efsevin-treated wild-type embryos). ***, p < 0.001 by one-way ANOVA. (E) Dose-dependence curve for efsevin. The tre embryos were treated with various concentrations of efsevin from 24 hpf and cardiac contractions were analyzed at 48 hpf. (F–H) Representative time traces of local field potentials for wild type (F), tre (G) and efsevin-treated tre (H) embryos clearly display periods of regular, irregular, and restored periodic electrical activity. (I) In vivo optical maps of Ca2+ activation represented by isochronal lines every 33 ms recorded from 36 hpf wild type (left), tre (center) and efsevin-treated tre (right) embryos.
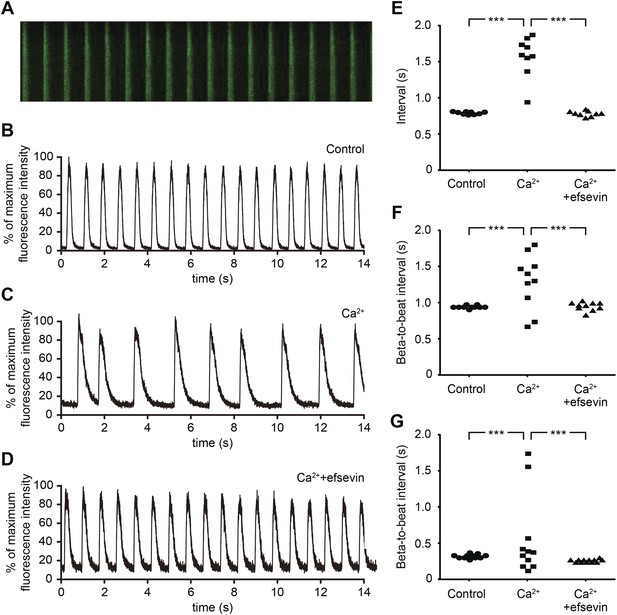
Efsevin reduces arrhythmogenic events in ES cell-derived cardiomyocytes.
(A) Line-scan analysis of Ca2+ transients in mESC-CMs after 10 days of differentiation. (B–D) Representative graph of Ca2+ transients detected in mESC-CMs (B). After treatment with 10 mM Ca2+ for 10 min, the EB showed an irregular pattern of Ca2+ transients (C). Efsevin treatment restores regular Ca2+ transients under Ca2+ overload conditions in mESC-CMs (D). (E) Plotted intervals between peaks of Ca2+ signals detected in mESC-CMs prior to treatment (control), in 10 mM Ca2+ext (Ca2+) and in 10 mM Ca2+ext+10 μM efsevin (Ca2++efsevin). (F, G) Plotted intervals of contractions detected in EBs prior to treatment (control), in 10 mM Ca2+ext (Ca2+) and in 10 mM Ca2+ext + 10 μM efsevin (Ca2+ + efsevin) for mouse ESC-CMs (F) and 5 mM Ca2+ext (Ca2+) and in 5 mM Ca2+ext + 5 μM efsevin (Ca2+ + efsevin) for human ESC-CMs (G). ***, p < 0.001 by F-test.
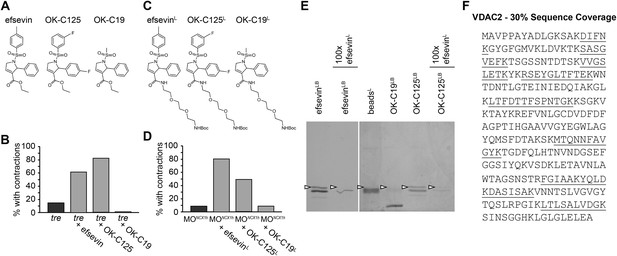
VDAC2 is a protein target of efsevin.
(A) Structures of efsevin and two derivatives, OK-C125 and OK-C19. (B) Efsevin and OK-C125 restored rhythmic contractions in the majority of tremblor embryos, whereas OK-C19 failed to rescue the tremblor phenotype. (C) Structures of linker-attached compounds (indicated by superscript L). (D) Compounds efsevinL and OK-C125L retained their ability to restore rhythmic contractions in NCX1hMO injected embryos, while the inactive derivative OK-C19L was still unable to induce rhythmic contraction. (E) Affinity agarose beads covalently linked with efsevin (efsevinLB) or OK-C125 (OK-C125LB) pulled down 2 protein species from zebrafish embryonic lysate, whereof one, the 32 kD upper band, was sensitive to competition with a 100-fold excess free efsevinL. The 32 kD band was not detected in proteins eluted from beads capped with ethanolamine alone (beadsC) or beads linked to OK-C19 (OK-C19LB). Arrowheads point to the 32kD bands. (F) Mass Spectrometry identifies the 32kD band as VDAC2. Peptides identified by mass spectrometry (underlined) account for 30% of the total sequence.
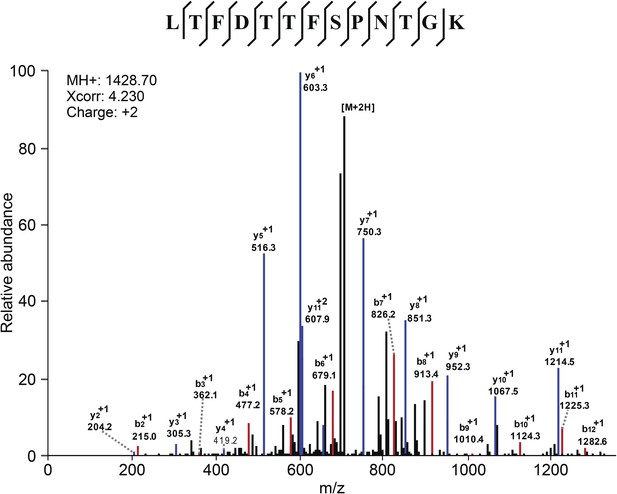
Mass Spectometry identifies VDAC2 as the target of efsevin.
Image shows an example of the identification of VDAC2 peptide. Diagnostic b- and y-series ions are shown in red and blue, respectively.
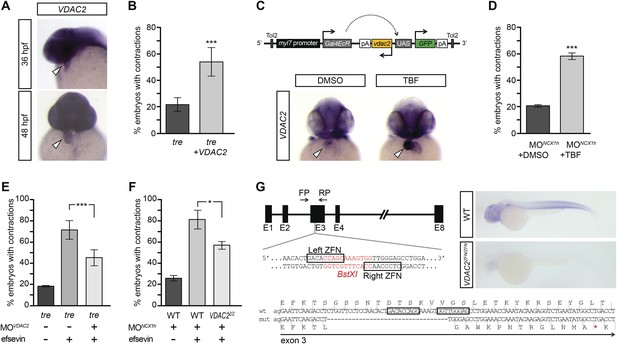
VDAC2 restores rhythmic cardiac contractions in tre.
(A) In situ hybridization analysis showed that VDAC2 is expressed in embryonic hearts at 36 hpf (upper image) and 48 hpf (lower image). (B) Injection of 25 pg in vitro synthesized VDAC2 mRNA restored cardiac contractions in 52.9 ± 12.1% (n = 78) of 1-day-old tre embryos, compared to 21.8 ± 5.1% in uninjected siblings (n = 111). (C) Schematic diagram of myl7:VDAC2 construct (top). In situ hybridization analysis showed that TBF treatment induces VDAC2 expression in the heart (lower panel). (D) While only ∼20% of myl7:VDAC2;NCX1hMO embryos have coordinated contractions (n = 116), 52.3 ± 2.4% of these embryos established persistent, rhythmic contractions after TBF induction of VDAC2 (n = 154). (E) On average, 71.2 ± 8.8% efsevin treated embryos have coordinated cardiac contractions (n = 131). Morpholino antisense oligonucleotide knockdown of VDAC2 (MOVDAC2) attenuates the ability of efsevin to suppress cardiac fibrillation in tre embryos (45.3 ± 7.4% embryos with coordinated contractions, n = 94). (F) Efsevin treatment restores coordinated cardiac contractions in 76.2 ± 8.7% NCX1MO embryos, only 54.1 ± 3.6% VDAC2zfn/zfn;NCX1MO embryos have coordinated contractions (n = 250). (G) Diagram of Zinc finger target sites. VDAC2zfn/zfn carries a 34 bp deletion in exon 3 which results in a premature stop codon (red asterisk). In situ hybridization analysis showing loss of VDAC2 transcripts in VDAC2zfn/zfn embryos. White arrowheads point to the developing heart.
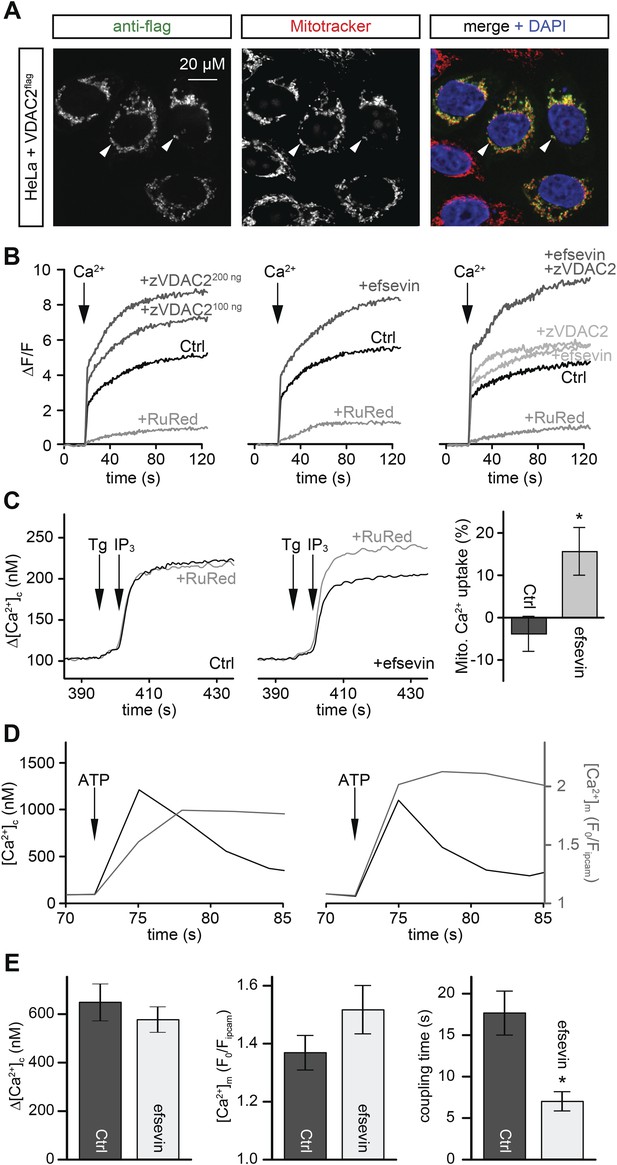
Efsevin enhances mitochondrial Ca2+ uptake.
(A) HeLa cells were transfected with a flag-tagged zebrafish VDAC2 (VDAC2flag), immunostained against the flag epitope and counterstained for mitochondria with MitoTracker Orange and for nuclei with DAPI. (B) Representative traces of mitochondrial matrix [Ca2+] ([Ca2+]m) detected by Rhod2. Arrows denote the addition of Ca2+. Mitochondrial Ca2+ uptake was assessed when VDAC2 was overexpressed (left), cells were treated with 1 µM efsevin (middle) and combination of both at suboptimal doses (right). Control-traces with ruthenium red (RuRed) show mitochondrial specificity of the signal. (C) Representative traces of cytosolic [Ca2+] ([Ca2+]c) changes upon the application of 7.5 µM IP3 in the presence (+) or absence (−) of RuRed. Mitochondrial Ca2+ uptake was assessed by the difference of the – and + RuRed conditions normalized to the total release (n = 4; mean ± SE). (D) MEFs overexpressing zebrafish VDAC2 (polycistronic with mCherry) were stimulated with 1 μM ATP in a nominally Ca2+ free buffer. Changes in [Ca2+]c and [Ca2+]m were imaged using fura2 and mitochondria-targeted inverse pericam, respectively. Black and gray traces show the [Ca2+]c (in nM) and [Ca2+]m (F0/F mtpericam) time courses in the absence (left) or present (right) of efsevin. (E) Bar charts: Cell population averages for the peak [Ca2+]c (left), the corresponding [Ca2+]m (middle), and the coupling time (time interval between the maximal [Ca2+]c and [Ca2+]m responses) in the presence (black, n = 24) or absence (gray, n = 28) of efsevin.
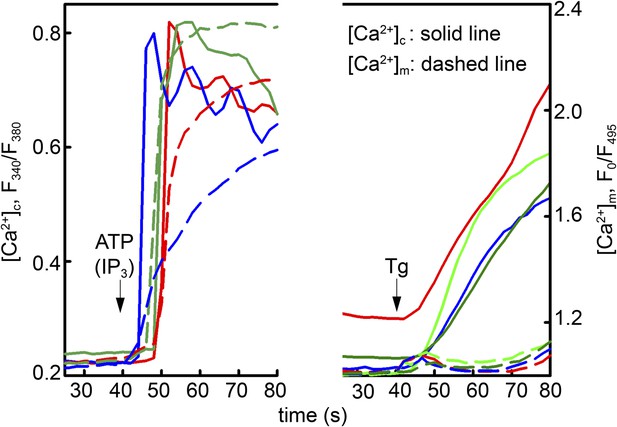
Local Ca2+ delivery between IP3 receptors and VDAC2.
V1V3DKO MEFs were stimulated with 100 μM ATP (left) or 2 μM thapsigargan (Tg) (right). Changes in [Ca2+]c and [Ca2+]m were imaged using fura2 and mitochondria targeted inverse pericam, respectively. Representative traces obtained in three cells are shown.
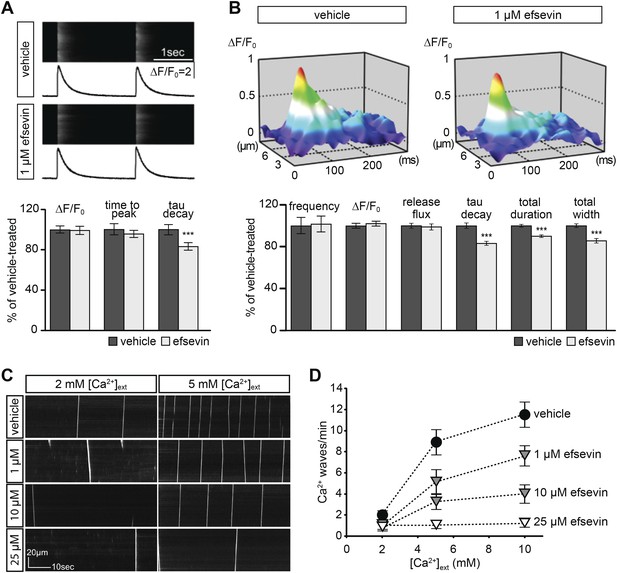
Effects of efsevin on isolated cardiomyocytes.
(A) Electrically paced Ca2+ transients at 0.5 Hz (top). Normalized quantification of Ca2+ transient parameters reveals no difference for transient amplitude (efsevin-treated at 98.6 ± 4.5% of vehicle-treated) and time to peak (95 ± 3.9%), but a significant decrease for the rate of decay (82.8 ± 4% of vehicle- for efsevin-treated) (lower panel). (B) Representation of typical Ca2+ sparks of vehicle- and efsevin treated cardiomyocytes (top). No differences were observed for spark frequency (101.1 ± 7.7% for efsevin- compared to vehicle-treated), maximum spark amplitude (101.6 ± 2.5%) and Ca2+ release flux (98.7 ± 2.8%). In contrast, the decay phase of the single spark was significantly faster in efsevin treated cells (82.5 ± 2.1% of vehicle-treated). Consequently, total duration of the spark was reduced to 85.7 ± 2% and the total width was reduced to 89.5 ± 1.4% of vehicle-treated cells. *, p < 0.05; ***, p < 0.001. (C) Increasing concentrations of extracellular Ca2+ induced a higher frequency of spontaneous propagating Ca2+ waves in isolated adult murine ventricular cardiomyocytes. Efsevin treatment reduced Ca2+ waves in a dose-dependent manner. (D) Quantitative analysis of spontaneous Ca2+ waves spanning more than half of the entire cell. Addition of 1 µM efsevin reduced Ca2+ waves to approximately half. Increasing the concentration of efsevin to 10 µM further reduced the number of spontaneous Ca2+ waves and 25 µM efsevin almost entirely blocked the formation of Ca2+ waves.
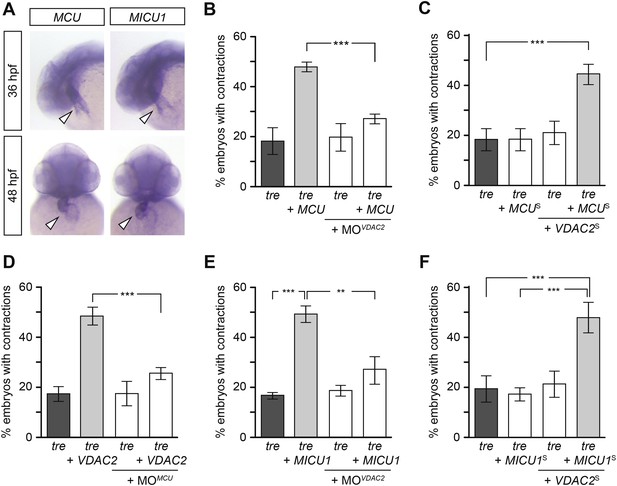
Mitochondria regulate cardiac rhythmicity through a VDAC2-dependent mechanism.
(A) MCU and MICU1 are expressed in the developing zebrafish hearts (arrowhead). (B) Overexpression of MCU is sufficient to restore coordinated cardiac contractions in tre embryos (47.1 ± 1.6% embryos, n = 112 as opposed to 18.3 ± 5.3% of uninjected siblings, n = 64) while this effect is significantly attenuated when co-injected with morpholino antisense oligonucleotide targeted to VDAC2 (27.1 ± 1.9% embryos, n = 135). (C) Suboptimal overexpression of MCU (MCUS) and VDAC2 (VDAC2S) in combination is able to suppress cardiac fibrillation in tre embryos (42.9 ± 2.6% embryos, n = 129). (D) The ability of VDAC2 to restore rhythmic contractions in tre embryos (48.5 ± 3.5% embryos, n = 111) is significantly attenuated when MCU is knocked down by antisense oligonucleotide (MOMCU) (25.6 ± 2.4% embryos, n = 115). (E) Overexpression of MICU1 is sufficient to restore rhythmic cardiac contractions in tre embryos (49.3 ± 3.4% embryos, n = 127 compared to 16.8 ± 1.4% of uninjected siblings, n = 150). This effect is abrogated by VDAC2 knockdown (MOVDAC2, 25.3 ± 5.5% embryos, n = 97). (F) Suboptimal overexpression of MICU1 (MICU1S) and VDAC2 (VDAC2S) in combination is able to restore rhythmic cardiac contractions in tre embryos (48.6 ± 6.0%, n = 106). Error bars represent s.d.; *p < 0.05; ***p < 0.001.
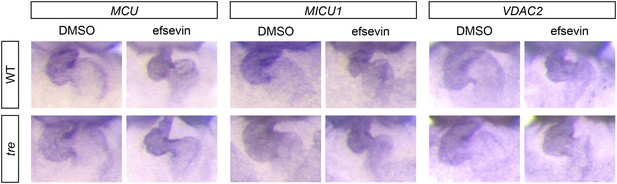
Expression of MCU, MICU1 and VDAC2.
In situ hybridization analysis shows that the expression levels of MCU, MICU1 and VDAC2 are comparable between wild type and tre embryos with and without efsevin treatment.
Videos
The video shows a heart of a wild-type zebrafish embryo at 2 dpf.
Robust rhythmic contractions can be observed in atrium and ventricle.
This video shows a heart of a tremblor embryo at 2 dpf.
Embryos of the mutant line tremblor display only local, unsynchronized contractions, comparable to cardiac fibrillation.
This video shows a heart of a tremblor embryo at 2 dpf treated with efsevin.
Treatment of tremblor embryos with efsevin restores rhythmic contractions with comparable atrial fractional shortening compared to wild-type embryos and approximately 40% of wild-type heart rate.
The video shows a heart of a wild-type zebrafish embryo at 2 dpf treated with efsevin.
Treatment of wild-type embryos with efsevin did not affect cardiac performance, indicated by robust, rhythmic contractions comparable to untreated wild-type embryos.
Heat map of Ca2+ transients recorded in 1 day old wild type heart.
https://doi.org/10.7554/eLife.04801.008Heat map of Ca2+ transients recorded in 1 day old tremblor heart.
https://doi.org/10.7554/eLife.04801.009Heat map of Ca2+ transients recorded in 1 day old efsevin treated tremblor heart.
https://doi.org/10.7554/eLife.04801.010This video shows a heart of a wild-type zebrafish embryo at 1 dpf.
Robust rhythmic contractions can be observed in atrium and ventricle.
This video shows a heart of a wild-type zebrafish embryo injected with zebrafish VDAC2 mRNA at 1 dpf.
Robust rhythmic contractions can be observed in atrium and ventricle.
This video shows a heart of a tremblor embryo at 1 dpf.
Tremblor embryos display only local, unsynchronized contractions, comparable to cardiac fibrillation.
This video shows a heart of a tremblor embryo injected with zebrafish VDAC2 mRNA at 1 dpf.
Overexpression of zebrafish VDAC2 mRNA restores rhythmic contractions in tremblor embryos.
This video shows a heart of a 2 dpf Tg-VDAC2 embryo injected with a morpholino targeting NCX1h.
Morpholino knock-down of NCX1h results in a fibrillating heart.
This video shows a heart of a 2 dpf NCX1h morphant in the Tg-VDAC2 genetic background.
TBF treatment induces VDAC2 expression and restores coordinated cardiac contractions.
This video shows a heart of a 2 dpf wild type zebrafish embryo injected with a morpholino targeting VDAC2.
Morpholino knockdown of VDAC2 did not have obvious effects on cardiac performance.
This video shows a heart of a 2 dpf tremblor mutant embryo injected with a morpholino targeting VDAC2.
https://doi.org/10.7554/eLife.04801.022This video shows a heart of a 2 dpf tremblor mutant embryo injected with a morpholino targeting VDAC2.
Efsevin treatment cannot restore coordinated cardiac contractions in the absence of VDAC2.





