Author Response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public review):
Expressed concern that FOOOF may not be sensitive to peaks located at the edges of the spectrum and suggested using rhythmicity as an alternative measure of oscillatory activity.
To address this concern, we first conducted a simulation in which we generated power spectra with a single periodic component while varying its parameters. The results confirmed that FOOOF may indeed have reduced sensitivity to low-frequency periodic components. In such cases, periodic activity can be conflated with aperiodic activity, leading to inflated estimates of the aperiodic component. These simulation results are presented in detail at the end of the Supplement.
To further investigate whether the low-frequency activity in our datasets may be oscillatory, we employed the phase-autocorrelation function (pACF), a measure of rhythmicity developed by Myrov et al. (2024). We compared pACF and FOOOF-derived parameters using linear mixed models at each channel–frequency– time point (see Methods for details). Our analyses showed that pACF activity closely resembles periodic activity across all three datasets, and is dissimilar to aperiodic parameters (see Figures 5, S4, S5, S21, S22, S34, S35). This supports the interpretation that, in our data, aperiodic activity is not conflated with periodic activity.
I was concerned that “there were no dedicated analyses in the paper to show that the aperiodic changes account for the theta changes.”
To address this concern, we used linear mixed models to estimate the association between FOOOF parameters and baseline-corrected time-frequency activity. These models were fitted at each channel-frequency-time point. Our results indicate that aperiodic activity is correlated with low-frequency (theta) baseline-corrected activity, while periodic activity is correlated primarily with activity in the alpha/beta range, but not with theta (see Figures 4, S3, S20, S33). Additionally, the exponent parameter exhibited a negative correlation in the gamma frequency range.
These findings support the reviewer's hypothesis: “I would also like to note that if the theta effect is only the aperiodic shift in disguise, we should see a concomitant increase in delta activity too – maybe even a decrease at high frequencies.” Overall, the results are consistent with our interpretation that low-frequency baseline-corrected activity reflects changes in aperiodic, rather than periodic, activity.
“On page 7 it is noted that baseline correction might subtract a significant amount of ongoing periodic activity. I would replace the word "subtract" with "remove" as not all baseline correction procedures are subtractive. Furthermore, while this sentence makes it sound like a problem, this is, to my mind, a feature, not a bug - baseline correction is meant to take away whatever is ongoing, be it oscillatory or not, and emphasise changes compared to that, in response to some event.”
We thank the reviewer for this helpful clarification. We have revised the sentence accordingly to read: “Our results show that classical baseline correction can remove continuous oscillatory activity that is present both during baseline and after stimulus onset, because it treats all baseline signals as 'background' to be removed without distinguishing between transient and continuous oscillations. While this is consistent with the intended purpose of baseline correction---to highlight changes relative to ongoing activity---it may also lead to unintended consequences, such as misinterpreting aperiodic activity as an increase in poststimulus theta oscillations.”
In addition, we have made several broader revisions throughout the manuscript to improve clarity and accuracy in response to the reviewer’s feedback:
(1) We have softened our interpretation of changes in the theta range. We no longer claim that these effects are solely due to aperiodic activity; rather, we now state that our findings suggest a potential contribution of aperiodic activity to signals typically interpreted as theta oscillations.
(2) We have revised our language to avoid suggesting a direct “interplay” between periodic and aperiodic components. Instead, we emphasize the concurrent presence of both components, using more precise and cautious formulations.
(3) We have clarified our discussion of baseline normalization approaches, explicitly noting that our findings hold regardless of whether a subtractive or divisive baseline correction was applied.
(4) Finally, we have restructured the introduction to improve readability and address points of potential confusion. Specifically, we have clarified the definition and role of 1/f activity, refined the discussion linking baseline correction to aperiodic activity, and improved transitions between key concepts.
Reviewer suggested that “it might be good to show that the findings were not driven by the cognitive-complaint subgroup (although the internal replications suggest they were not).”
We agree that it is important to demonstrate that our findings are not driven solely by the cognitive-complaint subgroup. While we did not include additional figures in the manuscript due to their limited relevance to the primary research question, we have attached figures that explicitly show the comparison between the clinical and control groups here in the response to reviewers. These figures include non-significant effects.
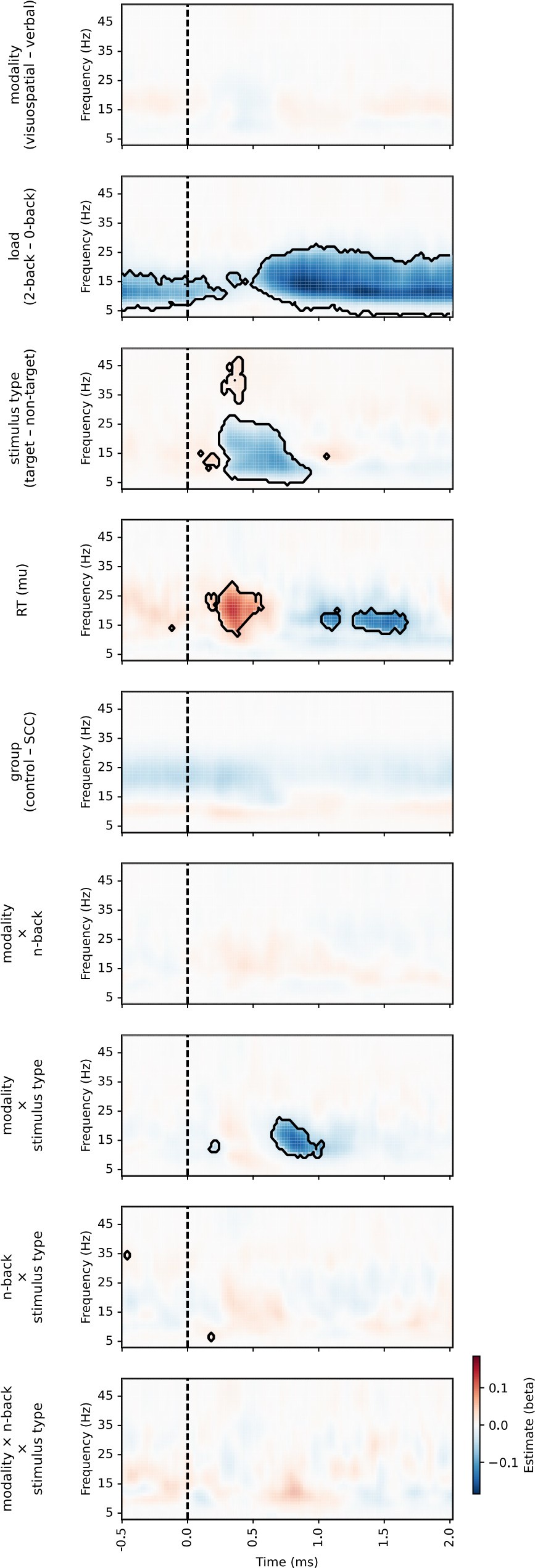
Author response image 1.
Results of the linear mixed model analysis of periodic activity for comparison between conditions, including non-significant effect (see also Figure 7 in the paper)
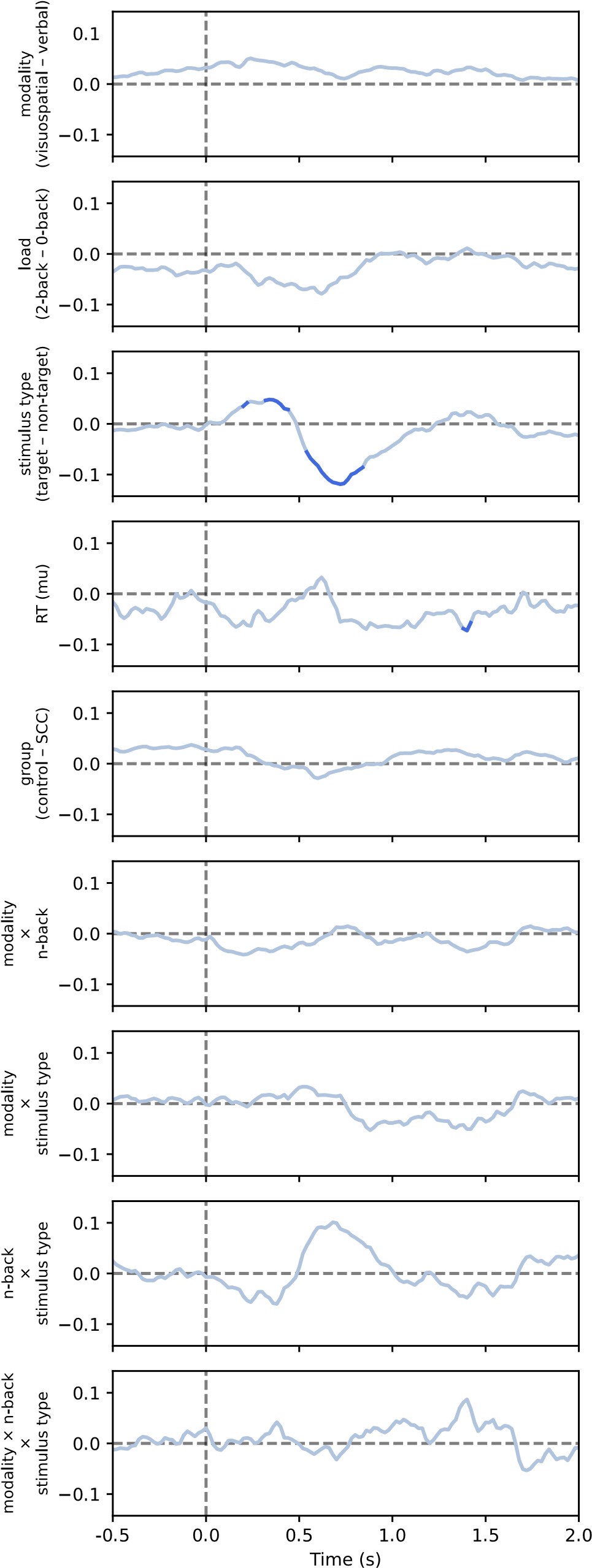
Author response image 2.
Results of the linear mixed model analysis of aperiodic exponent for comparison between conditions, including nonsignificant effects (see also Figure 9 in the paper)
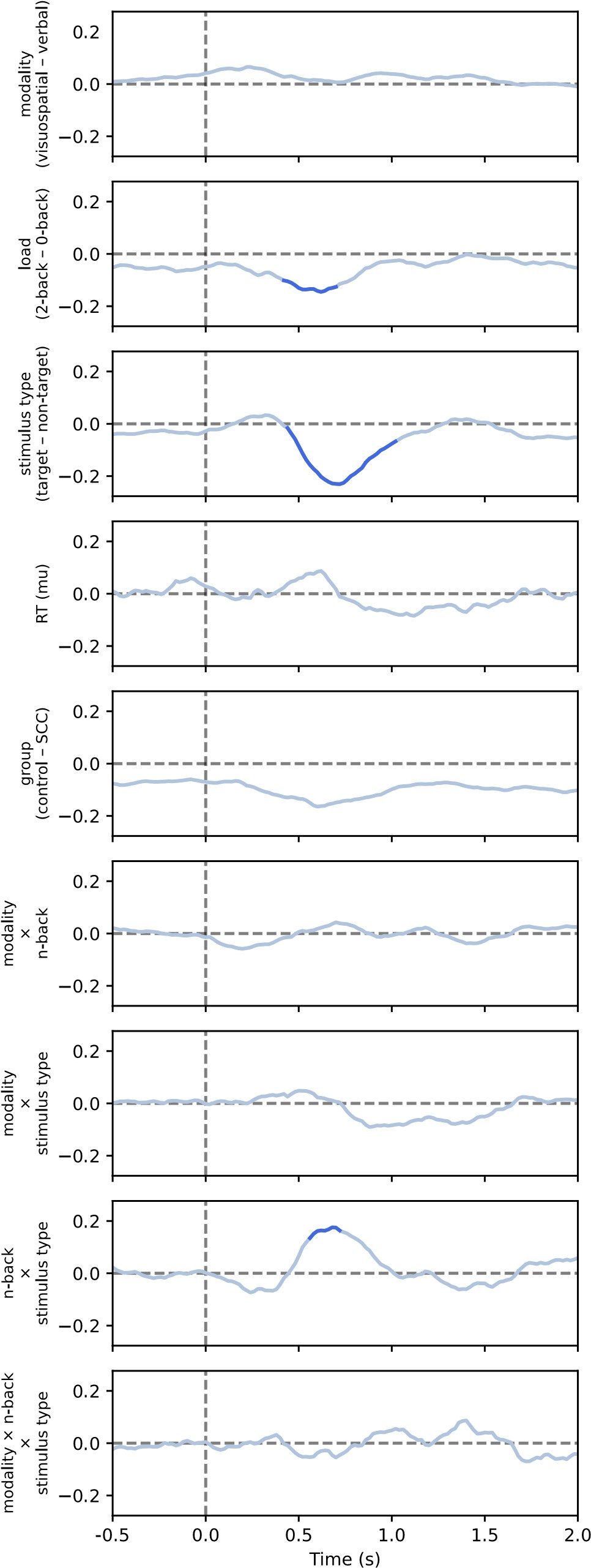
Author response image 3.
Results of the linear mixed model analysis of aperiodic offset for comparison between conditions, including non-significant effects (see also Figure S11 in the paper)
“Were lure trials discarded completely, or were they included in the non-target group?”
Thank you for the question. As described in the Methods section (EEG data preprocessing), lure trials were discarded entirely from further analysis and were not included in the non-target group.
“Also, just as a side note, while this time-resolved approach is definitely new, it is not novel to this paper, at least two other groups have tried similar approaches, e.g., Wilson, da Silva Castanheira, & Baillet, 2022; Ameen, Jacobs, et al., 2024.”
Thank you for drawing our attention to these relevant studies. We have now cited both Wilson et al. (2022) and Ameen et al. (2024) in our manuscript. While these papers did indeed use time-resolved approaches, to our knowledge our study is the first to use such an approach within a task-based paradigm.
noted that it was unclear how the periodic component was reconstructed: “I understand that a Gaussian was recreated based on these parameters, but were frequencies between and around the Gaussians just zeroed out? Or rather, given a value of 1, so that it would be 0 after taking its log10.”
The periodic component was reconstructed by summing the Gaussians derived from the FOOOF model parameters. Since the Gaussians asymptotically approach, but never reach, zero, there were no explicit zeros between them. We have included this explanation in the manuscript.
“If my understanding is correct, the periodic and aperiodic analyses were not run on the singletrial level, but on trial-averaged TF representations. Is that correct? In that case, there was only a single observation per participant for each within-subject cell at each TF point. This means that model (4) on p. 15 just simplifies to a repeated-measures ANOVA, does it not? As hinted at later in this section, the model was run at each time point for aperiodic analyses, and at each TF point for periodic analyses, resulting in a series of p-values or a map of p-values, respectively, is that correct?”
We thank the reviewer for this careful reading and helpful interpretation. The reviewer is correct that analyses were conducted on trial-averaged time-frequency representations. Model presented in equation 7 (as referred to in the current version of the manuscript) is indeed conceptually similar to a repeated-measures ANOVA in that it tests within-subject effects across conditions. However, due to some missing data (i.e., excluded conditions within subjects), we employed linear mixed-effects models (LMER), which can handle unbalanced data without resorting to listwise deletion. This provides more flexibility and preserves statistical power.
The reviewer is also correct that the models were run at each channel-time point for the aperiodic analyses, and at each channel-time-frequency point for the periodic analyses, resulting in a series or map of p-values, respectively.
suggested marking the mean response time and contrasting scalp topographies of response-related ERPs with those of aperiodic components.
We thank the reviewer for this helpful suggestion. In response, we have now marked the mean response time and associated confidence intervals on the relevant figures (Figures 8 and S8). Additionally, we have included a new figure (Figure S13) presenting both stimulus- and response-locked ERP scalp topographies for comparison with aperiodic activity.
In the previous version of the manuscript, we assessed the relationship between ERPs and aperiodic parameters by computing correlations between their topographies at each time point. However, to maintain consistency with our other analyses and to provide a more fine-grained view, we revised this approach and now compute correlations at each channel–time point. This updated analysis is presented in Figure S14. The results confirm that the correlation between ERPs and aperiodic activity remains low, and we discuss these findings in the manuscript.
Regardless of the low correlation, we have added the following statement to the manuscript to clarify our conceptual stance: “While contrasting response-related ERPs with aperiodic components can help address potential confounds, we believe that ERPs are not inherently separate from aperiodic or periodic activity. Instead, ERPs may reflect underlying changes in aperiodic and periodic activity. Therefore, different approaches to studying EEG activity should be seen as providing complementary rather than competing perspectives.”
“On page 3, it is noted that distinct theta peaks were only observed in 2 participants. Was this through visual inspection?”
Yes, this observation was based on visual inspection of the individual power spectra. We have included this explanation in the text.
suggested improving the plots by reducing the number of conditions (e.g., averaging across conditions), increasing the size of the colorbars, and using different color scales for different frequency bands, given their differing value ranges. Additionally, the reviewer noted that the theta and alpha results appeared surprising and lacked their expected topographical patterns, possibly due to the color scale.
We appreciate these thoughtful suggestions and have implemented all of them to improve the clarity and interpretability of the figures. Specifically, we reduced the number of conditions by averaging across them where appropriate, enlarged the colorbars for better readability, and applied separate color scales for different frequency bands to account for variability in dynamic range.
In the process, we also identified and corrected an error in the code that had affected the topographies of periodic activity in the previous version of the manuscript. With this correction, the resulting topographical patterns are now more consistent with canonical findings and are easier to interpret. For example, activity in the beta range now shows a clear central distribution (see Figure 6B and Figure S5B), and frontal activity in the theta range is more apparent.
This correction also directly addresses the reviewer’s concern that the “theta and alpha results (where visible) look surprising – the characteristic mid-frontal and posterior topographies, respectively, are not really present.” These unexpected patterns were primarily due to the aforementioned error.
“Relatedly, why is the mu parameter used here for correlations? Why not simply the RT mean/median, or one of the other ex-Gaussian parameters? Was this an a priori decision?”
We appreciate the reviewer's thoughtful question. While mean and median RTs are indeed commonly used as summary measures, we chose the mu parameter because it provides a more principled estimate of central tendency that explicitly accounts for the positive skew typically observed in RT distributions. Although we did not directly compare mu, mean and median in this dataset, our experience with similar datasets suggests that differences between them are typically small. We chose not to include other ex-Gaussian parameters (e.g., sigma, tau) to avoid unnecessary model complexity and potential overfitting, especially since our primary interest was not in modelling the full distribution of response variability. This decision was made a priori, although we note that the study was not pre-registered. We have now added a clarification in the manuscript to reflect this rationale.
“Relatedly, were (some) analyses of the study preregistered?”
The analyses were not preregistered. Our initial aim was to investigate differences in phaseamplitude coupling (PAC) between the clinical and control groups. However, we did not observe clear PAC in either group—an outcome consistent with recent concerns about the validity of PAC measures in scalp EEG data (see: https://doi.org/10.3390/a16120540). This unexpected finding prompted us to shift our focus toward examining the presence of theta activity and assessing its periodicity.
The reviewer suggested examining whether there might be differences between trials preceded by a target versus trials preceded by a non-target, potentially reflecting a CNV-like mechanism.
We appreciate the reviewer’s insightful suggestion. The idea of investigating differences between trials preceded by a target versus a non-target, possibly reflecting a CNV-like mechanism, is indeed compelling. However, this question falls outside the scope of the current study and was not addressed in our analyses. We agree that this represents an interesting direction for future research.
Reviewer #2 (Public review):
“For the spectral parameterization, it is recommended to report goodness-of-fit measures, to demonstrate that the models are well fit and the resulting parameters can be interpreted.”
We thank the reviewer for this suggestion. We have added reports of goodness-of-fit measures in the supplementary material (Fig. S9, S25, S41). However, we would like to note that our simulation results suggest that high goodness-of-fit values are not always indicative of accurate parameter estimation. For example, in our simulations, the R² values remained high even when the periodic component was not detectable or when it was conflated with the aperiodic component (e.g., compare Fig. S48 with Fig. S47). We now mention this limitation in the revised manuscript to clarify the interpretation of the goodness-of-fit metrics.
“Relatedly, it is typically recommended to set a maximum number of peaks for spectral parameterization (based on the expected number in the analyzed frequency range). Without doing so, the algorithm can potentially overfit an excessive number of peaks. What is the average number of peaks fit in the parameterized spectra? Does anything change significantly in setting a maximum number of peaks? This is worth evaluating and reporting.”
We report the average number of peaks, which was 1.9—2 (Figure S10). The results were virtually identical when setting number of peaks to 3.
“In the main text, I think the analyses of 'periodic power' (e.g. section ‘Periodic activity...’ and Figures 4 & 5 could be a little clearer / more explicit on the measure being analyzed. ‘Periodic’ power could in theory refer to the total power across different frequency bands, the parameterized peaks in the spectral models, the aperiodic-removed power across frequencies, etc. Based on the methods, I believe it is either the aperiodic power or an estimate of the total power in the periodic-only model fit. The methods should be clearer on this point, and the results should specify the measure being used.”
We thank the reviewer for highlighting this point. In our analyses, “periodic power” (or “periodic activity”) refers specifically to the periodic-only model fit. We have added clarifications under Figure 3 and in the Methods section to make this explicit in the revised manuscript.
“The aperiodic component was further separated into the slope (exponent) and offset components". These two parameters describe the aperiodic component but are not a further decomposition per se - could be rephrased.”
We thank the reviewer for alerting us to this potential misunderstanding. We have now rephrased the sentence to read: “The aperiodic component was characterised by the aperiodic slope (the negative counterpart of the exponent parameter) and the offset, which together describe the underlying broadband spectral shape.”
“In the figures (e.g. Figure 5), the channel positions do not appear to be aligned with the head layout (for example - there are channels that extend out in front of the eyes).”
Corrected.
“Page 2: aperiodic activity 'can be described by a linear slope when plotted in semi-logarithmic space'. This is incorrect. A 1/f distributed power spectrum has a linear slope in log-log space, not semi-log.”
Corrected.
Page 7: "Our results clearly indicate that the classical baseline correction can subtract a significant amount of continuous periodic activity". I am unclear on what this means - it could be rephrased.
We thank the reviewer to pointing out that the statement is not clear. We have now rephrased is to read: “Our results show that classical baseline correction can remove continuous oscillatory activity that is present both during baseline and after stimulus onset, because it treats all baseline signals as 'background' to be removed without distinguishing between transient and continuous oscillations.”
”Page 14: 'the FOOOF algorithm estimates the frequency spectrum in a semi-log space'. This is not quite correct - the algorithm parameterizes the spectrum in semi-log but does not itself estimate the spectrum.”
Again, we thank the reviewer for alerting us to imprecise description. We have now changed the sentence to: “The FOOOF algorithm parameterises the frequency spectrum in a semi-logarithmic space”.
We have made refinements to improve clarity, consistency, and flow of the main text. First, we streamlined the introduction by removing redundancies and ensuring a more concise presentation of key concepts. We also clarified our use of terminology, consistently referring to the ‘aperiodic slope’ throughout the manuscript, except where methodological descriptions necessitate the term ‘exponent.’ Additionally, we revised the final section of the introduction to better integrate the discussion of generalisability, ensuring that the inclusion of additional datasets feels more seamlessly connected to the study’s main objectives rather than appearing as an addendum. Finally, we carefully reviewed the entire manuscript to enhance coherence, particularly ensuring that discussions of periodic and aperiodic activity remain precise and do not imply an assumed interplay between the two components. We believe these revisions align with the reviewer’s suggestions and improve the overall readability and logical structure of the manuscript.
Reviewer #3 (Public review):
Raised concerns regarding the task's effectiveness in evoking theta power and the ability of our spectral parameterization method (specparam) to adequately quantify background activity around theta bursts.
We thank Reviewer #3 for their constructive feedback. To address the concerns regarding the task’s effectiveness in evoking theta power and the adequacy of our spectral parameterization method, we have added additional visualizations using a log-y axis ****(Figures S1, S19, S32). These figures demonstrate that, in baseline-corrected data, low-frequency activity during working memory tasks appears as both theta and delta activity. Additionally, we have marked the borders between frequency ranges with dotted lines to facilitate clearer visual differentiation between these bands. We believe these additions help clarify the results and address the reviewer’s concerns.
The reviewer noted that “aperiodic activity seems specifically ~1–2 Hz.”
In our data baseline-corrected low-frequency post-stimulus increase in EEG activity spans from approximately 3 to 7 Hz, with no prominent peak observed in the canonical theta band (4–7 Hz). While we did not analyze frequencies below 3 Hz, we agree with the reviewer that some of this activity could potentially fall within the delta range.
Nonetheless, we would like to emphasize that similar patterns of activity have often been interpreted as theta in the literature, even in the absence of a distinct spectral peak (see: https://doi.org/10.1016/j.neulet.2012.03.076; https://doi.org/10.1016/j.brainres.2006.12.076; https://doi.org/10.1111/psyp.12500; https://doi.org/10.1038/s42003-023-05448-z — particularly, see the interpretation of State 1 as a “theta prefrontal state”).
To accommodate both interpretations, we have opted to use the more neutral term “low-frequency activity” where appropriate. However, we also clarify that such activity is frequently referred to as “theta” in prior studies, even in the absence of a clear oscillatory peak.
“Figure 4 [now Figure 6]: there is no representation of periodic theta.”
Yes, this is one of the main findings of our study - periodic theta is absent in the vast majority of participants. A similar finding was found in a recent preprint on a working memory task (https://doi.org/10.1101/2024.12.16.628786), which further supports our results.
“Figure 5 [now Figure 7]: there is some theta here, but it isn't clear that this is different from baseline corrected status-quo activity.”
This figure shows comparisons of periodic activity between conditions. Although there are differences between conditions in the theta band, this does not indicate the presence of theta oscillations. Instead, the differences between the conditions in the theta band are most likely due to alpha components extending into the theta band (see Figure S6). This is further supported by the large overlap of significant channels between theta and alpha in Figure 7.
“Figure 8: On the item-recognition task, there appears to be a short-lived burst in the high delta / low theta band, for about 500 ms. This is a short phenomenon, and there is no evidence that specparam techniques can resolve such time-limited activity.”
We thank the reviewer for their comment. As we noted in our preliminary response, specparam, in the form we used, does not incorporate temporal information; it can be applied to any power spectral density (PSD), regardless of how the PSD is derived. Therefore, the ability of specparam to resolve temporal activity depends on the time-frequency decomposition method used. In particular, the performance of specparam is limited by the underlying time-frequency decomposition method and the data available for it. In fact, Wilson et al. (2022, https://doi.org/10.7554/eLife.77348), who have developed an approach for timeresolved estimation of aperiodic parameters, actually compare two approaches that differ only in their underlying time-frequency estimation method, while the specparam algorithm is the same in both cases. For the time-frequency decomposition we used superlets (https://doi.org/10.1038/s41467-020-20539-9), which have been shown to resolve short bursts of activity more effectively than other methods. To our knowledge, superlets provide the highest resolution in both time and frequency compared to wavelets or STFT.
To improve the stability of the estimates, we performed spectral parameterisation on trial-averaged power rather than on individual trials (unlike the approach in Wilson et al., 2022). In contrast, Gyurkovics et al. (2022) who also investigated task-related changes in aperiodic activity, estimated power spectra at the single-trial level, but stabilised their estimates by averaging over 1-second time windows; however, this approach reduced their temporal resolution. We have now clarified this point in the manuscript.
“The authors note in the introduction that ‘We hypothesised that the aperiodic slope would be modulated by the processing demands of the n-back task, and that this modulation would vary according to differences in load and stimulus type.’. This type of parametric variation would be a compelling test of the hypothesis, but these analyses only included alpha and beta power (Main text & Figure 4)”
We appreciate the reviewer's comment, but would like to clarify that the comparison between conditions was performed separately for both periodic power and aperiodic parameters. The periodic power analyses included all frequencies from 3 to 50 Hz (or 35 Hz in the case of the second dataset). All factors were included in the linear model (see LMM formula in equation 7 - subsection Methods / Comparisons between experimental conditions), but the figures only include fixed effects that were statistically significant. For example, Figure 7 shows the periodic activity and Figure 9 shows the exponent, with further details provided in other supplementary figures.
“Figure 5 does show some plots with some theta activity, but it is unclear how this representation of periodic activity has anything to do with the major hypothesis that aperiodic slope accounts for taskevoked theta.” /…/ In particular, specparam is a multi-step model fitting procedure and it isn't impressively reliable even in ideal conditions (PMID: 38100367, 36094163, 39017780). To achieve the aim stated in the title, abstract, and discussion, the authors would have to first demonstrate the robustness of this technique applied to these data.
We acknowledge these concerns and have taken several steps to clarify the relationship between the aperiodic slope and low-frequency activity, and to assess the robustness of the specparam (FOOOF) approach in our data.
First, we directly compared baseline-corrected activity with periodic and aperiodic components in all three data sets. These analyses showed that low-frequency increases in baseline-corrected signals consistently tracked aperiodic parameters - in particular the aperiodic exponent - rather than periodic theta activity (see Figs 4, S3, S20, S33). Periodic components, on the other hand, were primarily associated with baseline corrected activity in the alpha and beta bands. The aperiodic exponent also showed negative correlations with high beta/gamma baseline-corrected activity, which is exactly what would be expected in the case of a shift in the aperiodic slope (rather than delta/theta oscillations). See also examples at https://doi.org/10.1038/s41593-020-00744-x (Figures 1c-iv) or https://doi.org/10.1111/ejn.15361 (Figures 3c,d).
Next, because reviewer #1 was concerned that FOOOF might be insensitive to peaks at the edges of the spectrum, we ran a simulation that confirmed this concern. We then applied an alternative phase-based measure of oscillatory activity: the phase-autocorrelation function (pACF; Myrov et al., 2024). This method does not rely on spectral fitting and is sensitive to phase rather than amplitude. Across all datasets, pACF results were in close agreement with periodic estimates from FOOOF and were not correlated with aperiodic parameter estimates (Figs 5, S4, S5, S21, S22, S34, S35).
Taken together, these complementary analyses suggest that the apparent low-frequency (delta, theta) activity observed in the baseline-corrected data is better explained by changes in the aperiodic slope than by true low-frequency oscillations. While we acknowledge the limitations of any single method, the convergence between the techniques increases our confidence in this interpretation.
“How did the authors derive time-varying changes in aperiodic slope and exponent in Figure 6 [now Figure 8]?”
We thank the reviewer for this question. As explained in the Methods section, we first performed a time-frequency decomposition, averaged across trials, and then applied a spectral decomposition to each time point.
“While these methodological details may seem trivial and surmountable, even if successfully addressed the findings would have to be very strong in order to support the rather profound conclusions that the authors made from these analyses, which I consider unsupported at this time:
(a) ‘In particular, the similarities observed in the modulation of theta-like activity attributed to aperiodic shifts provide a crucial validation of our conclusions regarding the nature of theta activity and the aperiodic component.’
(b) ‘where traditional baseline subtraction can obscure significant neural dynamics by misrepresenting aperiodic activity as theta band oscillatory activity’
(d) ‘our findings suggest that theta dynamics, as measured with scalp EEG, are predominantly a result of aperiodic shifts.’
(e) ‘a considerable proportion of the theta activity commonly observed in scalp EEG may actually be due to shifts in the aperiodic slope’.
(f) ‘It is therefore essential to independently verify whether the observed theta activity is genuinely oscillatory or primarily aperiodic’
[this would be great, but first we need to know that specparam is capable of reliably doing this].”
We believe that our claims are now supported by the aforementioned analyses, namely associations between baseline-corrected time-frequency activity and FOOOF parameters and associations between FOOOF parameters and PACF.
The reviewer found it unclear what low-frequency phase has to do with 1/f spectral changes: ‘Finally, our findings challenge the established methodologies and interpretations of EEG-measured crossfrequency coupling, particularly phase-amplitude coupling’
We thank the reviewer for their comment. To address this concern, we have added further clarification in the Discussion section. Our results are particularly relevant for phase-amplitude coupling (PAC) based on theta, such as theta-gamma coupling. PAC relies on the assumption that there are distinct oscillations at both frequencies. However, if no clear oscillations are present at these frequencies— specifically, if theta oscillations are absent—then the computation of PAC becomes problematic.






