Figures and data
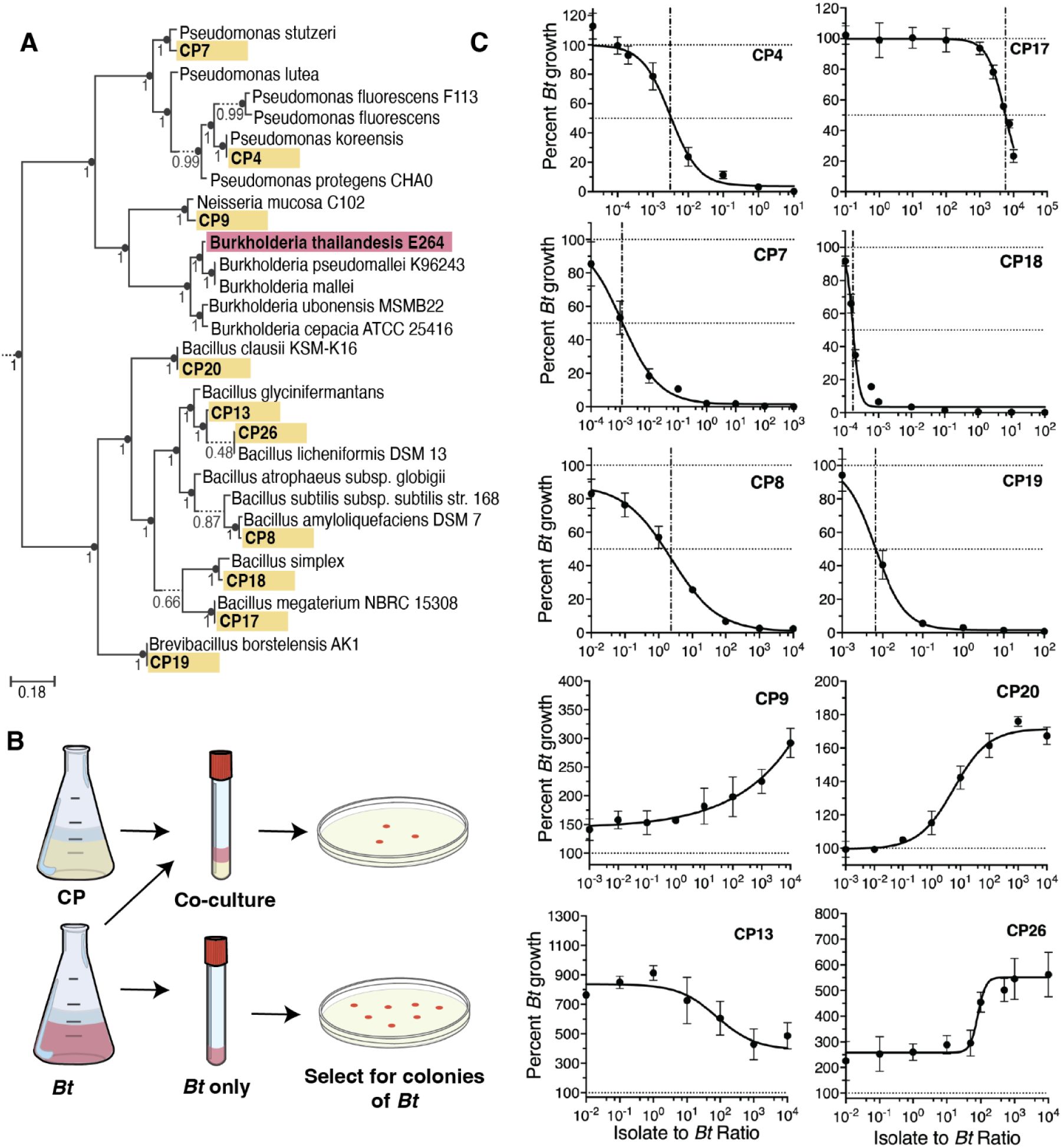
Characterization of CP activity against B. thailandensis in vitro.
(A) Phyloge nomic tree of CPs (yellow) and model pathogen B. thailandensis (Bt) (red), interspersed amongst publicly available genomes (no color). (B) Schematic of competition assay used to test in vitro inhibitory capabilities. Percent growth of Bt is calculated as the CFU in co-culture at 24 hours divided by the CFU in the Bt monoculture at 24 hours multiplied by 100. Co-culture inhibition was tested for multiple starting densities of CP (see Figure 1C x-axis). Starting densi ty of Bt and CP at a 1:1 ratio are 3.33×104 CFU/ml per organism. Bt monoculture is inoculated at 3.33×104 CFU/ml. (C) Dose-response curves for CPs against Bt. Relative IC50 is denoted with a vertical dashed-dotted line, and 100% and 50% growth are denoted with horizontal dotted lines. Percent growth at each density is represented as mean± SEM.
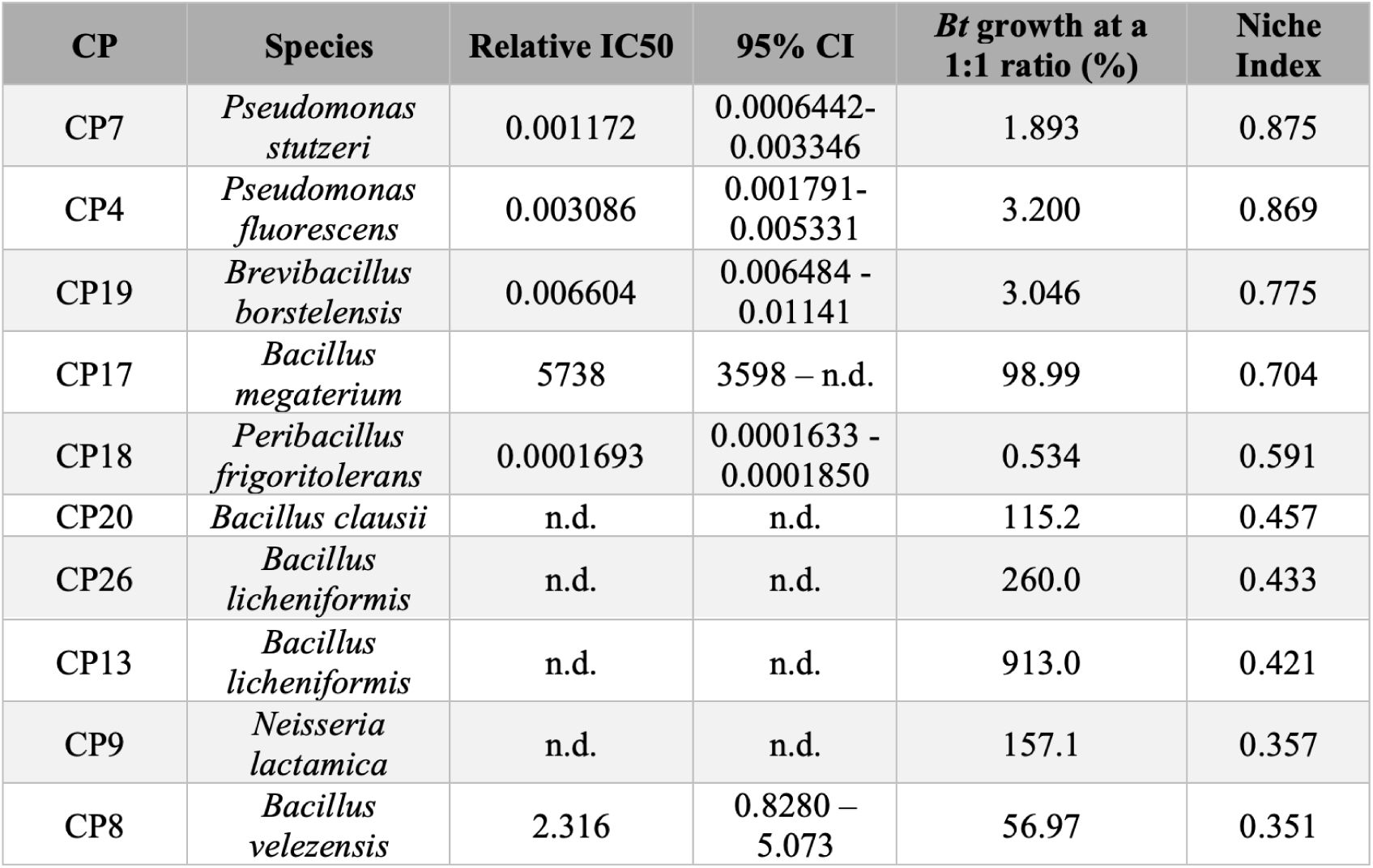
Summary of pathogen inhibition by CPs.
Relative IC50 values and 95% confidence intervals {95% Cl) are shown for each inhibitory CP. Not determined (n.d.) indicates that a value could not be measured. CPs are listed in order of decreasing NI values.
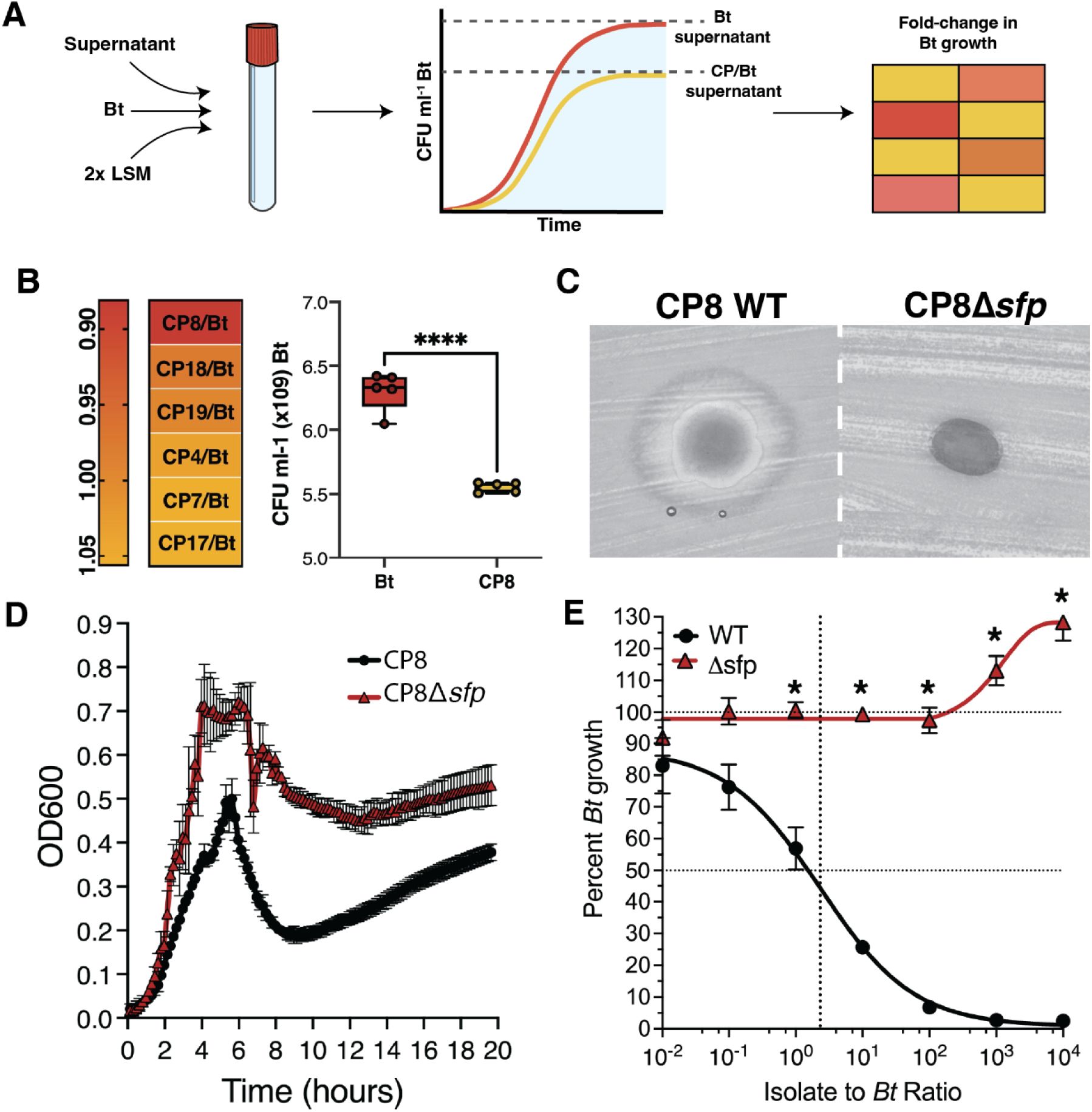
Production of a specialized metabolite plays a role in the antagonistic activities of CPS.
(A) Schematic representation of supernatant inhibition experiment workflow. Superna tant from a Bt and CP co-culture or from Bt monoculture (supernatant) is mixed 1:1 with 2x LSM. This mixture is used as the growth medium for subsequent Bt growth curves. Fold-change in Bt growth is calculated by dividing the maximum growth of Bt in co-culture supernatant by the maximum growth of Bt grown in supernatant from itself. (B) Left: Heatmap of fold-change in max CFU of Bt grown in supernatant from each inhibitory CP and Bt co-cul ture relative to Bt grown in supernatant from itself. Co-culture supernatant was collected at 72 hours. A fold-change of 1 indicates no difference in growth in co-culture supernatant compared to Bt growth in supernatant from itself. A fold-change less than 1 indicates a reduction in patho gen growth. Right: Boxplot of growth inhibition by CP8 supernatant. Data are represented as mean± SD. (C) Agar diffusion assays testing the CP8 wildtype strain (CP8 WT) and sfp mutant strain (CP8!:1sfp) for inhibition of Bt after 48 hours. (D) Growth curves of CP8 WT (black) and CP8!:1sfp (red) (E) Dose-response curve of CP8 WT (black) and CP8!:1sfp (red) against Bt after 24 hours of co-culture. Percent growth at each density is represented as mean ± SEM.
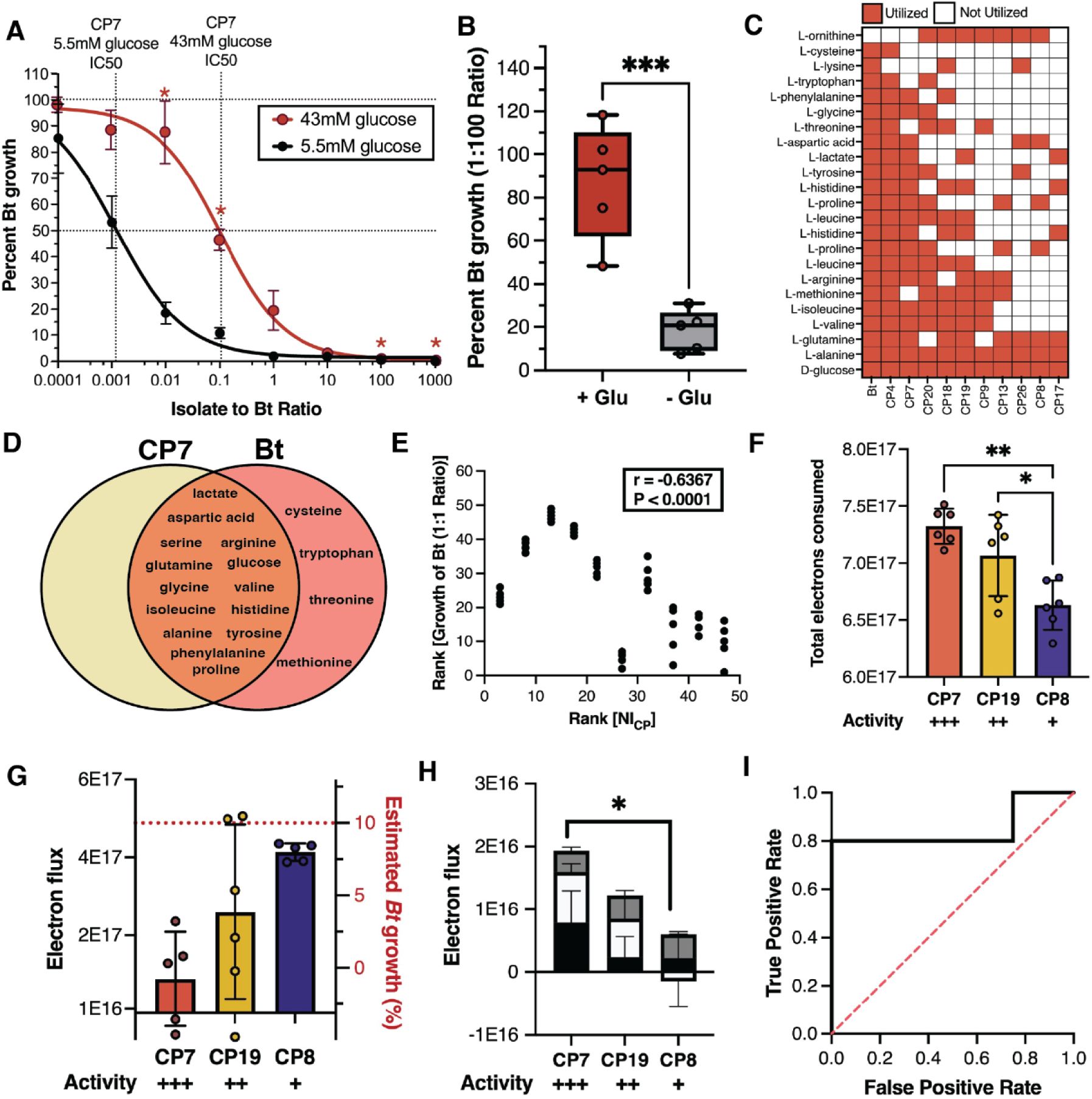
Metabolic niche overlap is indicative of pathogen and CP antagonism.
(A) Dose-response curve for CP7 grown in unsupplemented LSM (black) or with additional glucose (red). Percent growth at each density is represented as mean ± SEM. (B) Activity of CP7 against Bt at a 1:100 ratio with or without glucose supplementation (red and gray, respec tively). Data are summarized as mean ± SD. ***P<0.001. (C) Carbon utilization heatmap for CPs and Bt. (D) Venn diagram of carbon sources utilized by CP7 and Bt. (E) Plot of rank percent Bt growth at a 1:1 ratio after 24 hours vs rank of Niche Index for each CP. Correlation is determined by Spearman rank correlation (r = −0.6367, P < 0.0001, 95% Cl [−0.7891 −0.4261], N = 49). (F) Total carbon consumed by CPs grown in combination with Bt at 36 hours (mean ± SD). Activity indicates relative amount of Bt inhibition at a 1:1 ratio from most(+++) to least(+) inhibitory. *P=0.05; **P<0.01. (G) Total electron flux at 24 hours. Red dashed line indicates the estimated abundance of Bt in each co-culture. (H) Summed electron flux of lactate (black), praline (white), and aspartic acid (gray) at 24 hours in different CP co-culture conditions (mean± SD). *P<0.05.(I) Receiver operator characteristic curve showing the ability of growth on lactate to distinguish inhibitory CPs from non-inhibitory CPs. An AUROC of 1 indi cates perfect distinction between inhibitors and non-inhibitors; an AUROC of 0.5 (dashed red line) indicates no distinction between inhibitors and non-inhibitors.
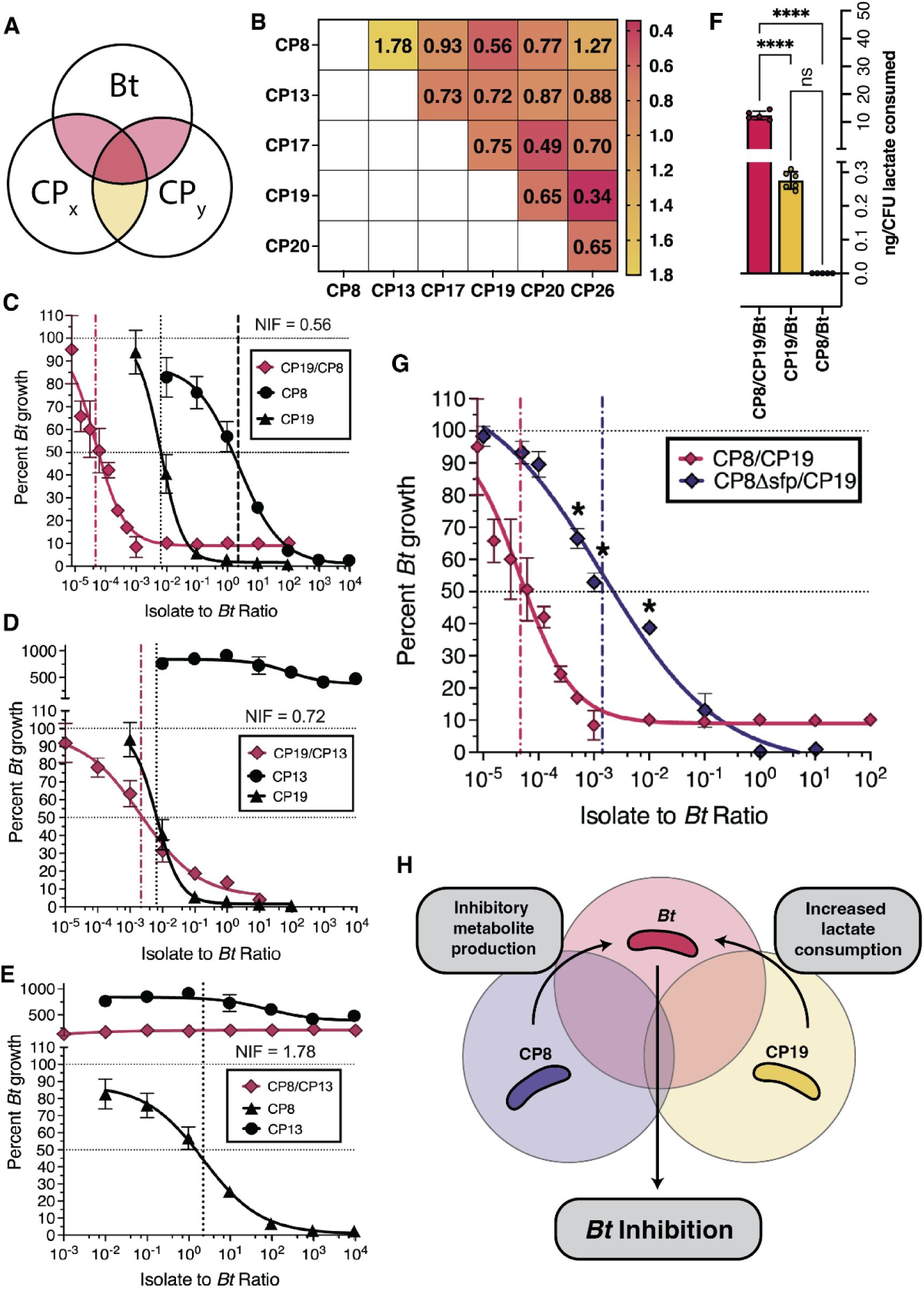
Niche overlap aids in identification of efficacious CP combinations.
(A) Venn diagram representa tion of the Niche Index Fraction (NIF) calculation. Yellow represents the fraction numerator and red the denomi nator. (B) Heatmap of NIF values for each colonizing CP. (C) Dose-response curve for the CP8/CP19 combina tion (mean ± SEM). (D) Dose-response curve for the CP19/CP13 combination (mean ± SEM). (E) Dose-re sponse curve for the CP13/CP8 combination (mean± SEM). (F) Lactate consumed (as determined via GC-TOF) for 3 conditions, normalized to CFU of each CP inoculated (mean ± SD). ****P<0.0001. (G) Dose-response curve for the CP8/CP19 (red) and CP8sfp/CP19.Llsfp (black) combinations. (H) Schematic of the proposed mechanism for Bt inhibition by the CP8/CP19 combination. CP8 produces a specialized metabolite that inhibits growth of the Bt. Additionally, increased lactate consumption by CP19 in the presence of CP8 further reduces Bt growth. Overall, minimal niche overlap between the CPs, and high overlap with Bt, allows for further inhibition of Bt with minimal inter-GP antagonism.
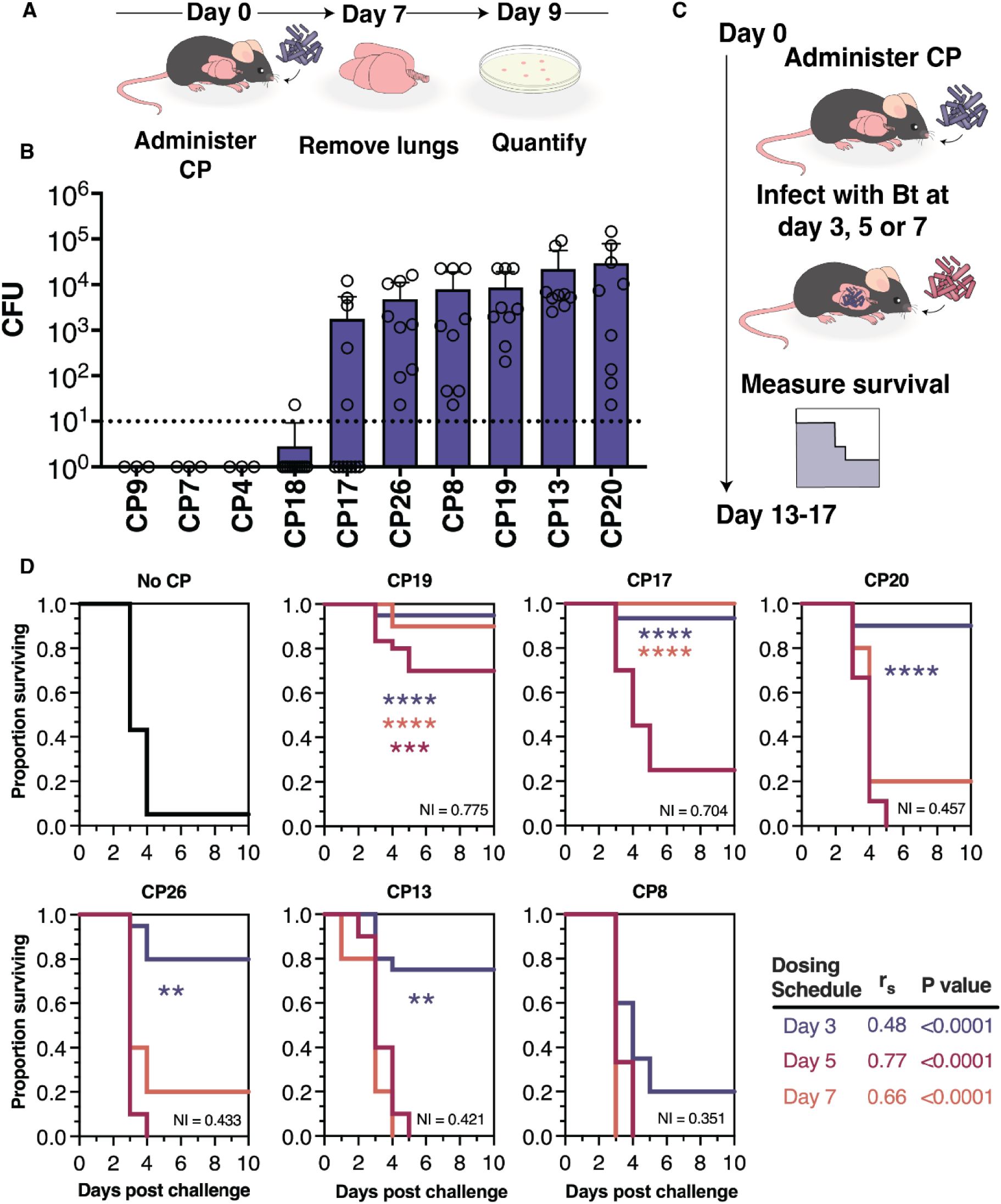
CPs colonize the mouse airway, and protect against respiratory Bt infection.
(A) Schematic of colo nization testing for CPs. Each CP (106 CFU) is administered to the lower airway via OPA.. After 7 days, the airway tissues (lungs and trachea) are collected, homogenized, and plated in order to enumerate viable CPs (expressed as CFU per tissue homogenate). (B) Colonization results from airway tissues collected at 7 days following CP administration. The dotted line represents the minimum CFU at which the CP is considered able to colonize in a reliably detectable manner. Data are represented as the mean ± SD. (C) Schematic of the method for survival testing. Each CP (106 CFU) is administered to the lower airway via OPA; and then, at 3, 5, or 7 days post-CP, chal lenged with a normally lethal dose of Bt (3×104 - 5×105 CFU). Survival is measured over the course of 10 days post-Bf. (D) Survival data from mice administered no CP (vehicle control), CPs at 3 days (blue), 5 days (orange), or 7 days (red) prior to Bt challenge. The Niche Index value for each CP is listed in the lower-right corner of its associated graph. Comparison of survival rate with versus without CP treatment was accomplished using the Mantel-Cox test ****P<0.0001, ***P<0.0002, **P<0.01. A summary of the relationship between days surviving and Niche Index value for each dosing schedule is also shown.
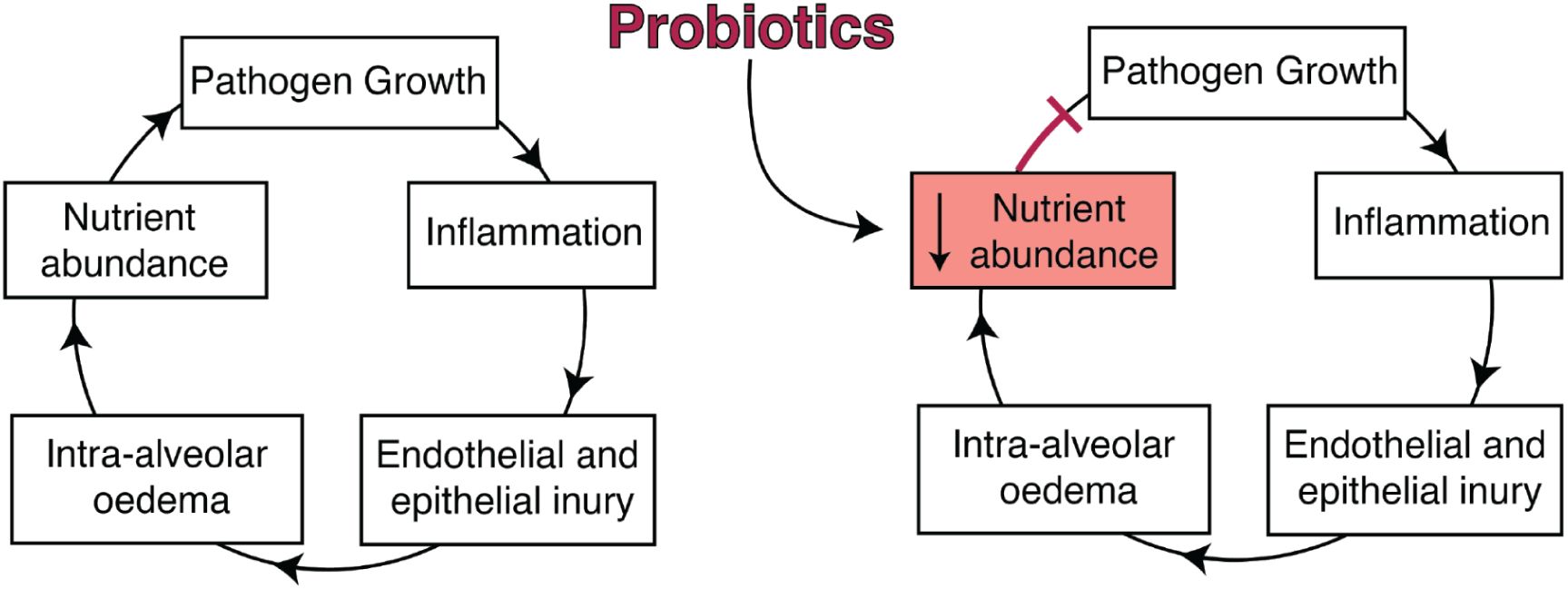
Proposed mechanism of CP protection.
Left: During lower airway infection, bacterial growth elicits inflammation from the host, with concurrent endothelial and epithelial injury. Intra-alveolar oedema introduces additional nutrients from the blood into the alveoli and lung lumen, enabling further growth of the pathogen. This positive feedback loop results in uncontrolled growth of the pathogen, ultimately facilitating its instantiation. Right: Probiotics delivered directly to the lower airway may limit infection by reducing nutrient abundance after oedema, which prevents further growth of the pathogen and thereby breaks the infection-promoting feedback loop.