Author response:
Description of the planned revisions
Reviewer #1 (Evidence, reproducibility and clarity):
Summary
The authors focused on medaka retinal organoids to investigate the mechanism underlying the eye cup morphogenesis. The authors succeeded to induce lens formation in fish retinal organoids using 3D suspension culture with minimal growth factor-containing media containing the Hepes. At day 1, Rx3:H2B-GFP+ cells appear in the surface region of organoids. At day 1.5, Prox1+cells appear in the interface area between the organoid surface and the core of central cell mass, which develops a spherical-shaped lens later. So, Prox1+ cells covers the surface of the internal lens cell core. At day 2, foxe3:GFP+ cells appear in the Prox1+ area, where early lens fiber marker, LFC, starts to be expressed. In addition, foxe3:GFP+ cells show EdU+ incorporation, indicating that foxe3:GFP+ cells have lens epithelial cell-characters. At day 4, cry:EGFP+ cells differentiate inside the spherical lens core, whose the surface area consists of LFC+ and Prox1+ cells. Furthermore, at day 4, the lens core moves towards the surface of retinal organoids to form an eye-cup like structure, although this morphogenesis "inside out" mechanism is different from in vivo cellular "outside -in" mechanism of eye cup formation. From these data, the authors conclude that optic cup formation, especially the positioning of the lens, is established in retinal organoids though the different mechanism of in vivo morphogenesis.
Overall, manuscript presentation is nice. However, there are still obscure points to understand background mechanism. My comments are shown below.
Major comments
(1) At the initial stage of retinal organoid morphogenesis, a spherical lens is centrally positioned inside the retinal organoids, by covering a central lens core by the outer cell sheet of retinal precursor cells. I wonder if the formation of this structure may be understood by differential cell adhesive activity or mechanical tension between lens core cells and retinal cell sheet, just like the previous study done by Heisenberg lab on the spatial patterning of endoderm, mesoderm and ectoderm (Nat. Cell Biol. 10, 429 - 436 (2008)). Lens core cells may be integrated inside retinal cell mass by cell sorting through the direct interaction between retinal cells and lens cells, or between lens cells and the culture media. After day 1, it is also possible to understand that lens core moves towards the surface of retinal organoids, if adhesive/tensile force states of lens core cells may be change by secretion of extracellular matrix. I wonder if the authors measure physical property, adhesive activity and solidness, of retinal precursor cells and lens core cells. If retinal organoids at day 1 are dissociated and cultured again, do they show the same patterning of internal lens core covering by the outer retinal cell sheet?
The question, whether different adhesive activity is involved in cell sorting and lens formation is indeed very intriguing. To address this point, we will include additional experiment (see Revision Plan, experiment 1). This experiment will be based on the dissociation and re-aggregation of lens-forming organoids as suggested by the reviewer. To monitor cell type specific sorting, we will employ a lens progenitor reporter line Foxe3::GFP and the retina-specific Rx2::H2B-RFP. If different adhesive activities of lens and retinal progenitor cells are involved and drive the process of cell sorting, dissociation and re-aggregation will result in cell sorting based on their identity.
(2) Optic cup is evaginated from the lateral wall of neuroepithelium of the diencephalon. In zebrafish, cell movement occurs from the pigment epithelium to the neural retina during eye morphogenesis in an FGF-dependent manner. How the medaka optic cup morphogenesis is coordinated? I also wonder if the authors conduct the tracking of cell migration during optic cup morphogenesis to reveal how cell migration and cell division are regulated in lens of the Medaka retinal organoids. It is also interesting to examine how retinal cell movement is coordinated during Medaka retinal organoids.
Looking into the detail of how optic cup-looking tissue arrangement of ocular organoids is achieved on cellular level is of course interesting. Our previous study showed that optic vesicles of medaka retinal organoids do not form optic cups (for details please see Zilova et al., 2021, eLIFE). We assume that the formation of cup-looking structure of the ocular organoids is mediated by the following processes: establishment of retina and lens domains at the specific region of the organoid – retina on the surface and lens in the center (see Figure S2 d and Figure 3e, and Figure 4). Further dislocation of the centrally formed lens towards the organoid periphery through the retina layer, places the lens to the periphery while retinal cells stay static. We assume that the “cup-like” shape is acquired by extrusion of the lens from the center of the organoid. To clarify this process with respect to tissue rearrangements and cell movements, we will include additional experiments (see Revision Plan, experiment 2) and follow lens- and retina-fated cells (by employing lens-specific Foxe3::GFP and retina-specific Rx2::H2B-RFP reporter lines) through the process of lens extrusion to dissect individual contribution of retinal/lens cells to this process (cross-reference with Reviewer #2).
(3) The authors showed that blockade of FGF signaling affects lens fiber differentiation in day 1-2, whereas lens formation seems to be intact in the presence of FGF receptor inhibitor in day 0-1. I suggest the authors to examine which tissue is a target of FGF signaling in retinal organoids, using markers such as pea3, which is a downstream target of ERK branch of FGF signaling. Since FGF signaling promotes cell proliferation, is the lens core size normal in SU5402-treated organoids from day 0 to day 1?
Assessing the activity of FGF signaling (cross-reference to Reviewer #3) in the organoids is indeed an important point. To address which tissue is the target of FGF signaling we will include additional experiments and assess the phosphorylation status of ERK (pERK) and expression of the ERK downstream target pea3, as suggested by the reviewer (see Revision Plan, experiment 3). That will allow to identify the tissue within the organoid responding to the Fgf signaling.
Lens core size of organoids treated with SU5402 from day 0 to day 1 is fully comparable to the control (please see Figure 6b).
(4) Fig. 3f and 3g indicate that there is some cell population located between foxe3:GFP+ cells and rx2:H2B-RFP+ cells. What kind of cell-type is occupied in the interface area between foxe3:GFP+ cells and rx2:H2B-RFP+ cells?
That is for sure an interesting question. We are aware of this population of cells. We currently do not have data that would with certainty clarify the fate of those cells. We are currently following up on that question with the use of scRNA sequencing, however we will not be able to address this question in the current manuscript.
(5) Fig. 5e indicates the depth of Rx3 expression at day 1. Is the depth the thickness of Rx3 expressing cell sheet, which covers the central lens core in the organoids? If so, I wonder if total cell number of Rx3 expressing cell sheet may be different in each seeded-cell number, because thickness is the same across each seeded-cell number, but the surface area size may be different depending on underneath the lens core size. Please clarify this point.
Yes. Figure 5e indicates the thickness of the cell sheet expressing Rx3 that lies on the surface of the organoid. Indeed, the number of Rx3-expressing cells (and lens cells) scales with the size of the organoid as stated in the submitted manuscript.
(6) Noggin application inhibits lens formation at day 0-1. BMP signaling regulates formation of lens placode and olfactory placode at the early stage of development. It is interesting to examine whether Noggin-treated organoid expands olfactory placode area. Please check forebrain territory markers.
What tissue differentiates at the expense of the lens in BMP inhibitor-treated organoids is of course an intriguing question. To address the identity of cells differentiated under this condition we will include an additional experiment (see Revision Plan, experiment 4 as suggested by the reviewer). We will check for the expression of Lhx2, Otx2 and Huc/D to address this point.
I have no minor comments
Referees cross-commenting
I agree that all reviewers have similar suggestions, which are reasonable and provided the same estimated time for revision.
Reviewer #1 (Significance):
Strength:
This study is unique. The authors examined eye cup morphogenesis using fish retinal organoids. Eye cup normally consists of the lens, the neural retina, pigment epithelium and optic stalk. However, retinal organoids seem to be simple and consists of two cell types, lens and retina. Interestingly, a similar optic cup-like structure is achieved in both cases; however, underlying mechanism is different. It is interesting to investigate how eye morphogenesis is regulated in retinal organoids,under the unconstrained embryo-free environment.
Limitation:
Description is OK, but analysis is not much profound. It is necessary to apply a bit more molecular and cellular level analysis, such as tracking of cell movement and visualization of FGF signnaling in organoid tissues.
Advancement:
The current study is descriptive. Need some conceptual advance, which impact cell biology field or medical science.
Audience:
The target audience of current study are still within ophthalmology and neuroscience community people, maybe translational/clinical rather than basic biology. To beyond specific fields, need to formulate a general principle for cell and developmental biology.
Reviewer #2 (Evidence, reproducibility and clarity):
In this study from Stahl et al., the authors demonstrate that medaka pluripotent embryonic cells can self-organise into eye organoids containing both retina and lens tissues. While these organoids can self-organize into an eye structure that resembles the vertebrate eye, they are built from a fundamentally different morphogenetic process – an “inside-out” mechanism where the lens forms centrally and moves outward, rather than the normal “outside-in” embryonic process. This is a very interesting discovery, both for our understanding of developmental biology and the potential for tissue engineering applications. The study would benefit from some additional experiments and a few clarifications.
The authors suggest that the lens cells are the ones that move from the central to a more superficial position. Is this an active movement of lens cells or just the passive consequence of the retina cells acquiring a cup shape? Are the retina cells migrating behind the lens or the lens cells pushing outwards? High-resolution imaging of organoid cup formation, tracking retina cells in combination with membrane labeling of all cells would help elucidate the morphogenetic processes occurring in the organoids. Membrane labeling would also be useful as Prox1 positive lens cells appear elongated in embryos while in the organoids, cell shapes seem less organised, less compact and not elongated (for example as shown in Fig 3f,g).
Looking into the detail of how optic cup-looking tissue arrangement of ocular organoids is achieved on cellular level is of course interesting. We assume that the formation of cup-looking structures of the ocular organoids is mediated by following processes: establishment of retina and lens domains at a specific region of the organoid – retina on the surface and lens in the center (see Figure S2 d and Figure 3e, and Figure 4). Further dislocation of centrally formed lenses towards the organoid periphery through the retina layer, place the lens to the periphery while retinal cells stay static. We assume that the “cup-like” shape is acquired by extrusion of the lens. To clarify this process with respect to tissue rearrangements and cell movements, we will include additional experiments (see Revision Plan, experiment 2). We will follow lens- and retina-fated cells (by employing lens-specific Foxe3::GFP and retina-specific Rx2::H2B-RFP reporter lines) through the process of lens extrusion to dissect the individual contribution of retinal/lens cells to this process (cross-reference with Reviewer #1).
The organoids could be a useful tool to address how cell fate is linked to cell shape acquisition. In the forming organoids, retinal tissue initially forms on the outside, while non-retinal tissue is located in the centre; this central tissue later expresses lens markers. Do the authors have any insights into why fate acquisition occurs in this pattern? Is there a difference in proliferation rates between the centrally located cells and the external ones? Could it be that highly proliferative cells give rise to neural retina (NR), while lower proliferating cells become lens?
The question how is the retinal and lens domain established in this specific manner is indeed intriguing and very interesting. We dedicated a part of the discussion to this topic. We discuss the role of the diffusion limit and the potential contribution of BMB and FGF signaling to this arrangement. Additional experiments (see Revision Plan, experiment 3) addressing the source and target tissues of FGF and BMP signaling in the organoid will ultimately bring more clarity to our understanding of the tissue arrangements in the organoid.
Although analysis of the proliferation rate of the cells at the surface and in the central region of the organoid might possibly show some differences in the proliferation rates between lens and retinal cells, we do not have any indications, that the proliferation rate itself would be instructive or superior to the cell fate decisions.
What happens in organoids that do not form lenses? Do these organoids still generate foxe3 positive cells that fail to develop into a proper lens structure? And in the absence of lens formation, does the retina still acquire a cup shape?
Lens formation is primarily dependent on acquisition/specification of Foxe3-expressing lens placode progenitors. If those are not present, a lens does not develop. Once Foxe3-expressing progenitors are established, a lens is formed in unperturbed conditions (measured by the presence of expression of crystallin proteins). In such conditions, organoids that do not have a lens, do not carry Foxe3-expressing cells.
In the absence of the lens, the organoid is composed of retinal neuroepithelium, that does not form an optic cup (for details of such phenotypes please see Zilova et al., 2021, eLIFE).
The author suggest that lens formation occurs even in the absence of Matrigel. Is the process slower in these conditions? Are the resulting organoids smaller? While there are indeed some LFC expressing cells by day2, these cells are not very well organised and the pattern of expression seems dotty. Moreover, LFC staining seems to localise posterior to the LFC negative, lens-like structure (e.g. Fig.S1 3o’clock).
How do these organoids develop beyond day 4? Do they maintain their structural integrity at later stages?
The role of HEPES in promoting organoid formation is intriguing. Do the authors have any insights into why it is important in this context? Have the authors tried other culture conditions and does culture condition influence the morphogenetic pathways occurring within the organoids?
We thank the reviewer for pointing this out. We were not clear in the wording and describing of our observation. Indeed, Matrigel is not required for acquisition of lens fate, which can be demonstrated with the expression of lens-specific markers. However, the presence of Matrigel has a profound impact on the structural aspects of organoid formation. Matrigel is essential for organization of retinal-committed cells into the retinal epithelium (Zilova et al., 2021, eLIFE). The absence of the structure of the retinal epithelium can indeed negatively impact on the cellular organization and the overall lens structure. To clarify the contribution of the Matrigel to the speed of organoid lens development and to the overall structure of the organoid lens we will perform additional experiments (see Revision Plan, experiment 5). With the use of Foxe3::GFP reporter line we will measure the onset of the lens-specific gene expression. In addition, we will use the immunohistochemistry to assess the gross morphology and size of the organoids grown without the Matrigel (cross-reference with Reviewer #3).
The role of the HEPES in lens formation is indeed very intriguing and currently under investigation. As HEPES is mainly used to regulate pH of the culture media and pH might have an impact on multiple cellular processes, it will require significant time investment to dissect molecular mechanism underlying the effect of HEPES on the process of lens formation (cross reference with Reviewer #3) and therefore cannot be addressed in the current manuscript.
Referees cross-commenting
Pleased to see that all the other reviewers are positive about the study and raise similar concerns and comments
Reviewer #2 (Significance):
This is a very interesting paper, and it will be important to determine whether this alternative morphogenetic process is specific to medaka or if similar developmental routes can be recapitulated in organoid cultures from other vertebrate species.
Reviewer #3 (Evidence, reproducibility and clarity):
Summary:
The manuscript by Stahl and colleagues reports an approach to generate ocular organoids composed of retinal and lens structures, derived from Medaka blastula cells. The authors present a comprehensive characterisation of the timeline followed by lens and retinal progenitors, showing these have distinct origins, and that they recapitulate the expression of differentiation markers found in vivo. Despite this molecular recapitulation, morphogenesis is strikingly different, with lens progenitors arising at the centre of the organoid, and subsequently translocating to the outside.
Comments:
- The manuscript presents a beautiful set of high quality images showing expression of lens differentiation markers over time in the organoids. The set of experiments is very robust, with high numbers of organoids analysed and reproducible data. The mechanism by which lens specification is promoted in these organoids is, however, poorly analysed, and the reader does not get a clear understanding of what is different in these experiments, as compared to previous attempts, to support lens differentiation. There is a mention to HEPES supplementation, but no further analysis is provided, and the fact that the process is independent of ECM contradicts, as the authors point out, previous reports. The manuscript would benefit from a more detailed analysis of the mechanisms that lead to lens differentiation in this setting.
The role of the HEPES in lens formation is indeed very intriguing and under current investigation. As HEPES is mainly used to regulate pH of the culture media and pH might have an impact on multiple cellular processes it will require a significant time investment to dissect molecular mechanism underlying the effect of HEPES on the process of lens formation (cross reference with Reviewer #2) and therefore unfortunately cannot be addressed in the current manuscript.
To clarify the contribution of the Matrigel to the organoid lens development we will perform additional experiments (see Revision Plan, experiment 5). With the use of Foxe3::GFP reporter line we will measure the onset of the lens-specific gene expression. In addition, we will use the immunohistochemistry to assess the gross morphology and size of the organoids grown without the Matrigel (cross-reference with Reviewer #2).
- The markers analysed to show onset of lens differentiation in the organoids seem to start being expressed, in vivo, when the lens placode starts invaginating. An analysis of earlier stages is not presented. This would be very informative, allowing to determine whether progenitors differentiate as placode and neuroepithelium first, to subsequently continue differentiating into lens and retina, respectively. Could early placodal and anterior neural plate markers be analysed in the organoids? This would provide a more complete sequence of lens vs retina differentiation in this model.
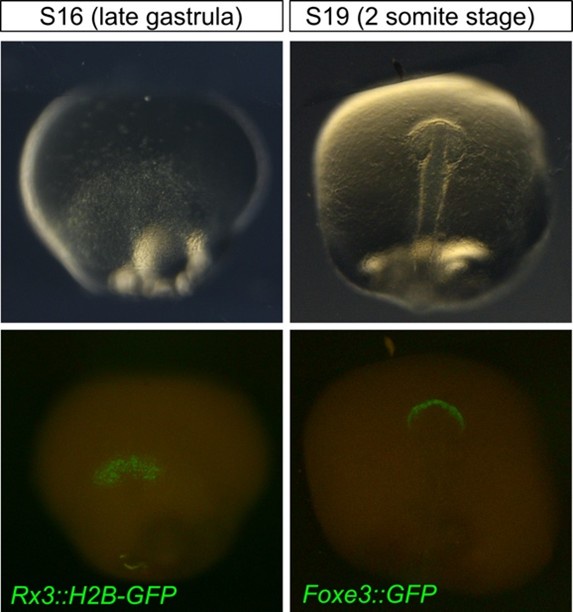
Yes. The figures show the expression of lens and retinal markers in the embryo in later developmental stages and the timing of their expression can be documented with higher temporal resolution. In the revised version of the manuscript, we will provide the information about the onset of expression of Rx3::H2B-GFP (retina) and Foxe3::GFP (lens) (see Author response image 1). Rx3 represents one of the earlies markers labeling the presumptive eye field within the region of the anterior neural plate (S16, late gastrula). FoxE3::GFP expression can be detected within the head surface ectoderm before the lens placode is formed showing that Foxe3 is a suitable marker of placodal progenitors in medaka.
We are convinced that the onset of Rx3 and Foxe3-driven reporters is early enough to make the claim about the separate origin of the lens (placodal) and retinal (anterior neuroectoderm) tissues within the ocular organoids.
Author response image 1.
- The analysis of BMP and Fgf requirement for lens formation and differentiation is suggestive, but the source of these signals is not resolved or mentioned in the manuscript. Are BMP4 and Fgf8 expressed by the organoids? Where are they coming from?
Indeed, addressing the source of BMP and FGF activation would bring more clarity in understanding the mechanism of retina/lens specification within the ocular organoids (cross reference with Reviewer #1). To address this point, we will include additional experiments (see Revision Plan, experiment 3). We will analyze the expression of respective ligands (Bmp4 and Fgf8) and activation of downstream effectors of BMP and FGF signaling pathways within the ocular organoids as suggested by Reviewer #1 and Reviewer #3.
- The fact that the lens becomes specified in the centre of the organoid is striking, but it is for me difficult to visualise how it ends up being extruded from the organoid. Did the authors try to follow this process in movies? I understand that this may be technically challenging, but it would certainly help to understand the process that leads to the final organisation of retinal and lens tissues in the organoid. There is no discussion of why the morphogenetic mechanism is so different from the in vivo situation. The manuscript would benefit from explicitly discussing this.
Following the extruding lens in vivo is indeed very relevant suggestion. To clarify the process of ocular organoid formation in the respect of tissue rearrangements and cell movements, we will include additional experiment (see Revision Plan, experiment 2). We will follow lens- and retina-fated cells (by employing lens-specific Foxe3::GFP and retina-specific Rx2::H2B-RFP reporter lines) through the process of lens extrusion (cross-reference with Reviewer #1 and Reviewer #2).
Referees cross-commenting
We all seem to have similar comments and concerns. I think overall the suggestions are feasible and realistic for the timeframe provided.
Reviewer #3 (Significance):
This study describes a reproducible approach to differentiate ocular organoids composed of lens and retinal tissues. The characterisation of lens differentiation in this model is very detailed, and despite the morphogenetic differences, the molecular mechanisms show many similarities to the in vivo situation. The manuscript however does not highlight, in my opinion, why this model may be relevant. Clearly articulating this relevance, particularly in the discussion, will enhance the study and provide more clarity to the readers regarding the significance of the study for the field of organoid research, ocular research and regenerative studies.
Revision Plan:
(1) To address whether differential adhesion properties of retinal and lens progenitors mediate cell sorting to establish retina and lens domains in the organoids (Reviewer #1, comment 1), we will perform dissociation of the organoids on day 1 and subsequential re-aggregation. This experiment will allow to follow cell type specific adhesion properties of lens and retinal progenitor cells. We will employ lens progenitor reporter line Foxe3::GFP and retina-specific Rx2::H2B-RFP to monitor cell type specific sorting with fluorescent microscopy.
(2) Multiple reviewers (Reviewer #1, Reviewer #2, Reviewer #3) asked for the presentation of detailed in vivo imaging experiment showing individual contributions of retina- and lens- fated cells to the resulting tissue organization withing the ocular organoid. We will perform in vivo live imaging experiment to follow the movements of individual lens (Foxe3::GFP) and retinal (Rx2::H2B-GFP) cells from day 1 to day 2 of organoid development to address this point.
(3) Reviewer #1 and Reviewer #3 raised questions concerning the role of FGF and BMP signaling and sources of these signaling pathway activities in ocular organoid tissue arrangement. To address this point and bring more light into the molecular mechanisms regulating lens and retina tissue arrangement in the organoid, we will perform additional experiment. We will assess the expression of candidate FGF and BMP ligands (Fgf8, Bmp7 and Bmp4) and activation of downstream effectors (p-ERK, p-SMAD) and the direct transcriptional target of Fgf signaling (Pea3) in the developing organoids. This will allow the identification of the tissue producing the ligand on one site and tissue responding to the signaling on the other site and help out to narrow down the molecular mechanism controlling tissue arrangements in the organoid.
(4) We will analyze the expression of forebrain territory markers in organoids treated with the BMP inhibitor to identify the identity of the tissue differentiating at the expense of lens under the BMP inhibition (suggested by Reviewer #1). We will label Noggin-treated organoids with the antibodies against Lhx2, Otx2 and HuC/D to address this point.
(5) We will provide more comprehensive analysis of the organoids grown without the Matrigel and compare them to the organoids grown in the presence of the Matrigel (mentioned by Reviewer #2 and Reviewer #3). With the use of lens progenitor-specific Foxe3::GFP reporter line, we will measure the onset of the lens-specific gene expression. In addition, we will use the immunohistochemistry to assess the gross morphology and size of the organoids grown without the Matrigel.
Description of analyses that authors prefer not to carry out
Reviewer #1:
(4) Fig. 3f and 3g indicate that there is some cell population located between foxe3:GFP+ cells and rx2:H2B-RFP+ cells. What kind of cell-type is occupied in the interface area between foxe3:GFP+ cells and rx2:H2B-RFP+ cells?
That is for sure interesting question. We are aware of this population of cells. We currently do not have a data that would with certainty clarify the fate of those cells. We are currently following up on that question with the use of scRNA sequencing, however we will not be able to address this question in the current manuscript.
Reviewer #2:
The role of HEPES in promoting organoid formation is intriguing. Do the authors have any insights into why it is important in this context? Have the authors tried other culture conditions and does culture condition influence the morphogenetic pathways occurring within the organoids?
The role of the HEPES in lens formation is indeed very intriguing and under current investigation. As HEPES is mainly used to regulate pH of the culture media and pH might have impact on multiple cellular processes it will require significant time investment to dissect molecular mechanism underlying the effect of the HEPES on the process of lens formation (cross reference with Reviewer #3) and cannot be addressed in the current manuscript.
Is there a difference in proliferation rates between the centrally located cells and the external ones? Could it be that highly proliferative cells give rise to neural retina (NR), while lower proliferating cells become lens?
Although analysis of the proliferation rate of the cells at the surface and in the central region of the organoid might possibly show some differences in the proliferation rates between lens and retinal cells, we do not have any indications, that the proliferation rate itself would be instructive or superior to the cell fate decisions.




