Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorRoy SillitoeBaylor College of Medicine, Houston, United States of America
- Senior EditorBarbara Shinn-CunninghamCarnegie Mellon University, Pittsburgh, United States of America
Reviewer #1 (Public Review):
The manuscript by Hariani et al. presents experiments designed to improve our understanding of the connectivity and computational role of Unipolar Brush Cells (UBCs) within the cerebellar cortex, primarily lobes IX and X. The authors develop and cross several genetic lines of mice that express distinct fluorophores in subsets of UBCs, combined with immunocytochemistry that also distinguishes subtypes of UBCs, and they use confocal microscopy and electrophysiology to characterize the electrical and synaptic properties of subsets of so-labelled cells, and their synaptic connectivity within the cerebellar cortex. The authors then generate a computer model to test possible computational functions of such interconnected UBCs.
Using these approaches, the authors report that:
GRP-driven TDtomato is expressed exclusively in a subset (20%) of ON-UBCs, defined electrophysiologically (excited by mossy fiber afferent stimulation via activation of UBC AMPA and mGluR1 receptors) and immunocytochemically by their expression of mGluR1.
UBCs ID'd/tagged by mCitrine expression in Brainbow mouse line P079 is expressed in a similar minority subset of OFF-UBCs defined electrophysiologically (inhibited by mossy fiber afferent stimulation via activation of UBC mGluR2 receptors) and immunocytochemically by their expression of Calretinin. However, such mCitrine expression was also detected in some mGluR1 positive UBCs, which may not have shown up electrophysiologically because of the weaker fluorophore expression without antibody amplification.
Confocal analysis of crossed lines of mice (GRP X P079) stained with antibodies to mGluR1 and calretinin documented the existence of all possible permutations of interconnectivity between cells (ON-ON, ON-OFF, OFF-OFF, OFF-ON), but their overall abundance was low, and neither their absolute or relative abundance was quantified.
A computational model (NEURON ) indicated that the presence of an intermediary UBC (in a polysynaptic circuit from MF to UBC to UBC) could prolong bursts (MF-ON-ON), prolong pauses (MF-ON-OFF), cause a delayed burst (MF-OFF-OFF), cause a delayed pause (MF-OFF-ON) relative to solely MF to UBC synapses which would simply exhibit long bursts (MF-ON) or long pauses (MF-OFF).
The authors thus conclude that the pattern of interconnected UBCs provides an extended and more nuanced pattern of firing within the cerebellar cortex that could mediate longer lasting sensorimotor responses.
The cerebellum's long known role in motor skills and reflexes, and associated disorders, combined with our nascent understanding of its role in cognitive, emotional, and appetitive processing, makes understanding its circuitry and processing functions of broad interest to the neuroscience and biomedical community. The focus on UBCs, which are largely restricted to vestibular lobes of the cerebellum reduces the breadth of likely interest somewhat. The overall design of specific experiments is rigorous and the use of fluorophore expressing mouse lines is creative. The data that is presented and the writing are clear. However, despite some additional analysis in response to the initial review, the overall experimental design still has issues that reduce overall interpretation (please see specific issues for details), which combined with a lack of thorough analysis of the experimental outcomes undermines the value of the NEURON model results and the advance in our understanding of cerebellar processing in situ (again, please see specific issues for details).
Specific issues:
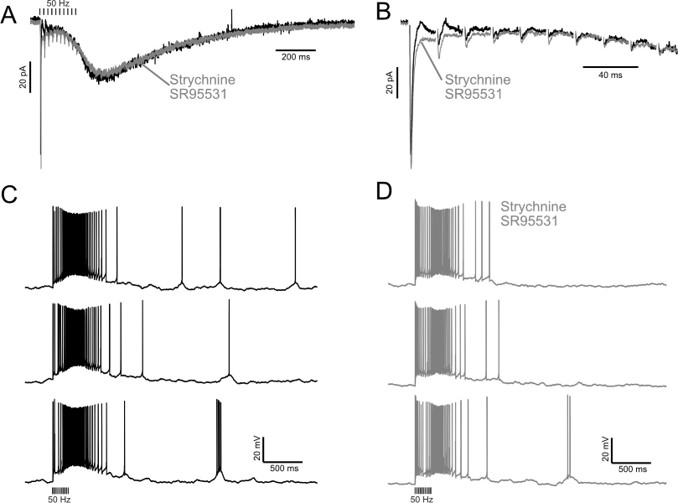
- All data gathered with inhibition blocked. All of the UBC response data (Fig. 1) was gathered in the presence of GABAAR and Glycine R blockers. While such an approach is appropriate generally for isolating glutamatergic synaptic currents, and specifically for examining and characterizing monosynaptic responses to single stimuli, it becomes problematic in the context of assaying synaptic and action potential response durations for long lasting responses, and in particular for trains of stimuli, when feed-forward and feed-back inhibition modulates responses to afferent stimulation. I.e. even for single MF stimuli, given the >500ms duration of UBC synaptic currents, there is plenty of time for feedback inhibition from Golgi cells (or feedforward, from MF to Golgi cell excitation) to interrupt AP firing driven by the direct glutamatergic synaptic excitation. This issue is compounded further for all of the experiments examining trains of MF stimuli. Beyond the impact of feedback inhibition on the AP firing of any given UBC, it would also obviously reduce/alter/interrupt that UBC's synaptic drive of downstream UBCs. This issue fundamentally undermines our ability to interpret the simulation data of Vm and AP firing of both the modeled intermediate and downstream UBC, in terms of applying it to possible cerebellar cortical processing in situ.
The authors' response to the initial concern is (to paraphrase), "its not possible to do and its not important", neither of which are soundly justified.
As stated in the original review, it is fully understandable and appropriate to use GABAAR/GlycineR antagonists to isolate glutamatergic currents, to characterize their conductance kinetics. That was not the issue raised. The issue raised was that then using only such information to generate a model of in situ behavior becomes problematic, given that feedback and lateral inhibition will sculpt action potential output, which of course will then fundamentally shape their synaptic drive of secondary UBCs, which will be further sculpted by their own inhibitory inputs. This issue undermines interpretation of the NEURON model.
The argument that taking inhibition into account is not possible because of assumed or possible direct electrical excitation of Golgi cells is confusing for two interacting reasons. First, one can certainly stimulate the mossy fiber bundle to get afferent excitation of UBCs (and polysynaptic feedback/lateral inhibitory inputs) without directly stimulating the Golgi cells that innervate any recorded UBC. Yes, one might be stimulating some Golgi cells near the stimulating electrode, but one can position the stimulating electrode far enough down the white matter track (away from the recorded UBC), such that mossy fiber inputs to the recorded UBC can be stimulated without affecting Golgi cells near or synaptically connected to the recorded UBC. Moreover, if the argument were true, then presumably the stimulation protocol would be just as likely to directly stimulate neighboring UBCs, which then drove the recorded UBC's responses. Thus, it is both doable and should be ensured that stimulation of the white matter is distant enough to not be directly activating relevant, connected neurons within the granule cell layer.
Finally, the authors present three examples of UBC recordings with and without inhibitory inputs blocked, and state "Thus, these large conductances are unlikely to be significantly shaped by 1-10 ms IPSCs from feedforward and feedback GABA/glycine inhibition" and "GABA/glycinergic inhibition...has little to no effect on the slow inward current that develops after the end of stimulation". This response reflects on original concerns about lack of quantification or consideration of important parameters. In particular, while the traces with and without inhibition are qualitatively similar, quantitative considerations indicate otherwise. First, unquantified examples are not adequate to drive conclusions. Regardless, the main issue (how inhibition affects actual responses in situ) is actually highlighted by the authors current clamp recordings of UBC responses, before and after blocking inhibition. The output response is dramatically different, both at early and late time points, when inhibition is blocked. Again, a lack of quantification (of adequate n's) makes it hard to know exactly how important, but quick "eye ball" estimates of impact include: 1) a switch from only low frequency APs initially (without inhibition blocked) to immediate burst of high frequency APs (high enough to not discern individual APs with given figure resolution) when inhibition is blocked, 2) Slow rising to a peak EPSP, followed by symmetrical return to baseline (without inhibition blocked) versus immediate rise to peak, followed by prolonged decay to baseline (with inhibition blocked), 3) substantially shorter duration (~34% shorter) secondary high frequency burst (individual APs not discernible) of APs (with inhibition blocked versus without inhibition blocked), and 4) substantial reduction in number of long delayed APs (with inhibition blocked versus without inhibition blocked). Thus, clearly, feedback/lateral inhibition is actually sculpting AP output at all phases of the UBC response to trains of afferent stimulations. Importantly, the single voltage clamp trace showing little impact of transient IPSCs on the slow EPSC do not take into account likely IPSC influences on voltage-activated conductances that would not occur in voltage-clamp recordings but would be free to manifest in current clamp, and thereby influence AP output, as observed.
So again, our ability to understand how interconnected UBCs behave in the intact system is undermined by the lack of consideration and quantification of the impact of inhibition, and it not being incorporated into the model. At the very least a strong proviso about lack of inclusion of such information, given the authors' data showing its importance in the few examples shown, should be added to the discussion.
- No consideration for involvement of polysynaptic UBCs driving UBC responses to MF stimulation in electrophysiology experiments. Given the established existence (in this manuscript and Dino et al. 2000 Neurosci, Dino et al. 2000 ProgBrainRes, Nunzi and Mugnaini 2000 JCompNeurol, Nunzi et al. 2001 JCompNeurol) of polysynaptic connections from MFs to UBCs to UBCs, the MF evoked UBC responses established in this manuscript, especially responses to trains of stimuli could be mediated by direct MF inputs, or to polysynaptic UBC inputs, or possibly both (to my awareness not established either way). Thus the response durations could already include extension of duration by polysynaptic inputs, and so would overestimate the duration of monosynaptic inputs, and thus polysynaptic amplification/modulation, observed in the NEURON model.
Author response: "UBCs receive a single mossy fiber input on their dendritic brush, and thus if our stimulation produces a reliable, short-latency response consistent with a monosynaptic input, then there is not likely to be a disynaptic input."
This statement is not congruent with the literature, with early work by Mugnaini and colleagues (Mugnaini et al. 1994 Synapse; Mugnaini and Flores 1994 J. Comp. Neurol.) indicating that UBCs are innervated by 1-2 mossy fibers, which are as likely other UBC terminals as MFs. This leaves open the possibility that so called monosynaptic responses do, as originally suggested, already include polysynaptic feedforward amplification of duration. While the authors also indicate that isolated disynaptic currents can be observed when they occur in isolation, a careful examination and objective documentation of "monosynaptic" responses would address this issue. Presumably, if potential disynaptic UBC inputs occur during a monosynaptic MF response, it would be detected as an abrupt biphasic inward/outward current, due to additional AMPA receptor activation but further desensitization of those already active (as observed by Kinney et al. 1997 J. Neurophysiol: "The delivery of a second MF stimulus at the peak of the slow EPSC evoked a fast EPSC of reduced amplitude followed by an undershoot of the subsequent slow current"). If such polysynaptic inputs are truly absent and are "rare" in isolation, some estimation of how common or not such synaptic amplification is, would improve our understanding of the overall significance of these inputs.
- Lack of quantification of subtypes of UBC interconnectivity. Given that it is already established that UBCs synapse onto other UBCs (see refs above), the main potential advance of this manuscript in terms of connectivity is the establishment and quantification of ON-ON, ON-OFF, OFF-ON, and OFF-OFF subtypes of UBC interconnections. But, the authors only establish that each type exists, showing specific examples, but no quantification of the absolute or relative density was provided, and the authors' unquantified wording explicitly or implicitly states that they are not common. This lack of quantification and likely small number makes it difficult to know how important or what impact such synapses have on cerebellar processing, in the model and in situ.
To address this issue, the authors added the following text to the discussion section: "We did not estimate the density of these UBC to UBC connections, because the sparseness of labeling using these approaches made an accurate calculation impossible. Previous work using organotypic slice cultures from P8 mice estimated that 2/3 of the UBC population receives input from other UBCs (Nunzi & Mugnaini, 2000), although it is unclear whether this is the case in older mice."
While accurate, the addition doesn't really address the situation, which is that apparently the reported connections are rare. Adding the information about 2/3 of UBCs having UBC inputs in culture, implies the opposite might be true (i.e. that they might be quite common), which is in contrast to the authors' data, so should be reworded for clarity, which should also incorporate the considerations covered in point #2 above. I.e. if the authors do establish that none of their recordings have polysynaptic inputs, and if they determine that the number of cells that showed isolated di-synaptic inputs is indeed rare, then it suggests that these specific polysynaptic connections are in fact rare.
- Lack of critical parameters in NEURON model.
A) The model uses # of molecules of glutamate released as the presumed quantal content, and this factor is constant. However, no consideration of changes in # of vesicles released from single versus trains of APs from MFs or UBCs is included. At most simple synapses, two sequential APs alters release probability, either up or down, and release probability changes dynamically with trains of APs. It is therefore reasonable to imagine UBC axon release probability is at least as complicated, and given the large surface area of contact between two UBCs, the number of vesicles released for any given AP is also likely more complex.
B) the model does not include desensitization of AMPA receptors, which in the case of UBCs can paradoxically reduce response magnitude as vesicle release and consequent glutamate concentration in the cleft increases (Linney et al. 1997 JNeurophysiol, Lu et al. 2017 Neuron, Balmer et al. 2021 eLIFE), as would occur with trains of stimuli at MF to ON-UBCs.
While the authors have not added the suggested additional parameters, their clarifications regarding the implications of existing parameters, and demonstration of reasonable fits to experimental data, and lack of substantial effect of simulating reduced vesicle release probability, provided by the authors, adequately addresses this concern.
- Lack of quantification of various electrophysiological responses. UBCs are defined (ON or OFF) based on inward or outward synaptic response, but no information is provided about the range of the key parameter of duration across cells, which seems most critical to the current considerations. There is a similar lack of quantification across cells of AP duration in response to stimulation or current injections, or during baseline. The latter lack is particularly problematic because in agreement with previous publications, the raw data in Fig. 1 shows ON UBCs as quiescent until MF stimulation and OFF UBCs firing spontaneously until MF stimulation, but, for example, at least one ON UBC in the NEURON model is firing spontaneously until synaptically activated by an OFF UBC (Fig. 11A), and an OFF UBC is silent until stimulated by a presynaptic OFF UBC (Fig. 11C). This may be expected/explainable theoretically, but then such cells should be observed in the raw data.
The authors have added additional analysis and discussion, which adequately addresses this concern.
Reviewer #2 (Public Review):
In this paper, the authors presented a compelling rationale for investigating the role of UBCs in prolonging and diversifying signals. Based on the two types of UBCs known as ON and OFF UBC subtypes, they have highlighted the existing gaps in understanding UBCs connectivity and the need to investigate whether UBCs target UBCs of the same subtype, different subtypes, or both. The importance of this knowledge is for understanding how sensory signals are extended and diversified in the granule cell layer.
The authors designed very interesting approaches to study UBCs connectivity by utilizing transgenic mice expressing GFP and RFP in UBCs, Brainbow approach, immunohistochemical and electrophysiological analysis, and computational models to understand how the feed-forward circuits of interconnected UBCs transform their inputs.
This study provided evidence for the existence of distinct ON and OFF UBC subtypes based on their electrophysiological properties, anatomical characteristics, and expression patterns of mGluR1 and calretinin in the cerebellum. The findings support the classification of GRP UBCs as ON UBCs and P079 UBCs as OFF UBCs and suggest the presence of synaptic connections between the ON and OFF UBC subtypes. In addition, they found that GRP and P079 UBCs form parallel and convergent pathways and have different membrane capacitance and excitability. Furthermore, they showed that UBCs of the same subtype provide input to one another and modify the input to granule cells, which could provide a circuit mechanism to diversify and extend the pattern of spiking produced by mossy fiber input. Accordingly, they suggested that these transformations could provide a circuit mechanism for maintaining a sensory representation of movement for seconds.
Overall, the article is well written in a sound detailed format, very interesting with excellent discovery and suggested model.
I believe the authors have provided appropriate responses and have consequently revised the manuscript in a convincing manner. Although I am not an expert in physiology, I find the explanations and clarifications to be acceptable.