Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorKate WassumUniversity of California, Los Angeles, Los Angeles, United States of America
- Senior EditorKate WassumUniversity of California, Los Angeles, Los Angeles, United States of America
Reviewer #1 (Public Review):
In the presence of predators, animals display attenuated foraging responses and increased defensive behaviors that serve to protect them from potential predatory attacks. Previous studies have shown that the basolateral nucleus of the amygdala (BLA) and the periaqueductal gray matter (PAG) are necessary for the acquisition and expression of conditioned fear responses. However, it remains unclear how BLA and PAG neurons respond to predatory threats when animals are foraging for food. To address this question, Kim and colleagues conducted in vivo electrophysiological recordings from BLA and PAG neurons and assessed approach-avoidance responses while rats search for food in the presence of a robotic predator.
The authors observed that rats exhibited a significant increase in the latency to obtain the food pellets and a reduction in the pellet success rate when the predator robot was activated. A subpopulation of PAG neurons showing increased firing rate in response to the robot activation didn't change their activity in response to food pellet retrieval during the pre- or post-robot sessions. Optogenetic stimulation of PAG neurons increased the latency to procure the food pellet in a frequency- and intensity-dependent manner, similar to what was observed during the robot test. Combining optogenetics with single-unit recordings, the authors demonstrated that photoactivation of PAG neurons increased the firing rate of 10% of BLA cells. A subsequent behavioral test in 3 of these same rats demonstrated that BLA neurons responsive to PAG stimulation displayed higher firing rates to the robot than BLA neurons nonresponsive to PAG stimulation. Next, because the PAG does not project monosynaptically to the BLA, the authors used a combination of retrograde and anterograde neural tracing to identify possible regions that could convey robot-related information from PAG to the BLA. They observed that neurons in specific areas of the paraventricular nucleus of the thalamus (PVT) that are innervated by PAG fibers contained neurons that were retrogradely labeled by the injection of CTB in the BLA. In addition, PVT neurons showed increased expression of the neural activity marker cFos after the robot test, suggesting that PVT may be a mediator of PAG signals to the BLA.
Overall, the idea that the PAG interacts with the BLA via the midline thalamus during a predator vs. foraging test is new and quite interesting. The authors have used appropriated tools to address their questions.
In this revised version of the manuscript, the authors have made important modifications in the text, inserted new data analyses, and incorporated additional references, as recommended by the reviewers. These modifications have significantly improved the quality of the manuscript.
Reviewer #2 (Public Review):
The authors characterized activity of the dorsal periaqueductal gray (dPAG) - basolateral amygdala (BLA) circuit. They show that BLA cells that are activated by dPAG stimulation are also more likely to be activated by a robot predator. These same cells are also more likely to display synchronous firing.
The authors also replicate prior results showing that dPAG stimulation evokes fear and the dPAG is activated by a predator.
Lastly, the report performs anatomical tracing to show that the dPAG may act on the BLA via the paraventricular thalamus (PVT). Indeed, the PVT receives dPAG projections and also projects to the BLA. However, the authors do not show if the PVT mediates dPAG to BLA communication with any functional behavioral assay. Furthermore, the authors also do not thoroughly characterize the activity of BLA cells during the predatory assay.
The major impact in the field would be to add evidence to their prior work, strengthening the view that the BLA can be downstream of the dPAG.
Reviewer #3 (Public Review):
In the present study, the authors examined how dPAG neurons respond to predatory threats and how dPAG and BLA communicate threat signals. The authors employed single-unit recording and optogenetics tools to address these issues in an 'approach food-avoid predator' paradigm. They characterized dPAG and BLA neurons responsive to a looming robot predator and found that dPAG opto-stimulation elicited fleeing and increased BLA activity. Importantly, they found that dPAG stimulation produces activity changes in subpopulations of BLA neurons related to predator detection, thus supporting the idea that dPAG conveys innate fear signals to the amygdala. In addition, injections of anterograde and retrograde tracers into the dPAG and BLA, respectively, along with the examination of c-FOS activity in midline thalamic relay stations, suggest that the paraventricular nucleus of the thalamus (PVT) may serve as a mediator of dPAG to BLA neurotransmission. Of relevance, the study helps to validate an important concept that dPAG mediates primal fear emotion and may engage upstream amygdalar targets to evoke defensive responses. The series of experiments provide a compelling case for supporting their conclusions. The study brings important concepts revealing dynamics of fear-related circuits particularly attractive to a broad audience, from basic scientists interested in neural circuits to psychiatrists.
Author response:
The following is the authors’ response to the previous reviews.
Recommendations for the authors:
We sincerely value the insightful and constructive feedback (italicized) provided by the reviewers, which has been instrumental in identifying areas of our manuscript that required further clarification or amendment. In response to these valuable comments, we have significantly revised the manuscript to enhance clarity and accuracy. Specifically, we have corrected an oversight related to the robot’s velocity and secondary antibody ratios, and addressed previously missing values in Figs. 3E and 4E. Importantly, these corrections did not alter the outcomes of our results. Additionally, we have enriched our manuscript with new data analyses, as reflected in Figures 1B, 1F, 2H-J, 4D, 4F-H, S1A, S1C-E, S3H, S5, and Table 1, ensuring a more comprehensive presentation of our findings. Below are our responses detailing each comment and explaining the modifications integrated into the revised manuscript.
Reviewer 1:
(1) To address the question of whether PAG photostimulation biases the cells that respond to the robot, a counterbalanced experiment, in which the BLA activity is initially recorded during the foraging vs. robot test and the PAG stimulation happens at the end of the session, should have been performed.
In our study, we investigated fear behavior and BLA cell responses to intrinsic dPAG photostimulation (320 pulses) in naïve animals, followed by their reactions to an extrinsic predatory robot. We recognize the reviewer's concern regarding the potential influence of initial dPAG photostimulation on BLA neuron responses to the robot. We address this issue in our discussion (pg. 13) as follows: “However, it is crucial to consider the recent discovery that optogenetic stimulation of CA3 neurons (3000 pulses) leads to gain-of-function changes in CA3-CA3 recurrent (monosynaptic) excitatory synapses (Oishi et al., 2019). Although there is no direct connection between dPAG neurons and the BLA (Vianna and Brandao 2003, McNally, Johansen, and Blair 2011, Cameron et al. 1995), and no studies have yet demonstrated gain-of-function changes in polysynaptic pathways to our knowledge, the potential for our dPAG photostimulation (320 pulses) to induce similar changes in amygdalar neurons, thereby enhancing their sensitivity to predatory threats, cannot be dismissed.”
(2) In Figure 3, it is unclear which criteria (e.g. response latency, minimum Z score, spike fidelity) was used to identify the BLA neurons that were indirectly activated by PAG stimulation. A graphic containing at least the distribution of the response latencies for each BLA neuron after PAG laser activation is needed.
We have specified the criteria for determining the responsiveness of BLA neurons to dPAG stimulation on page 22. This involves analyzing the first 500-ms post-stimulation across five 0.1-s bins. Units were classified as ‘stim cells’ if they showed z-scores greater than 3 (z > 3) in any of the bins during the initial 500-ms period post-stimulation. Neurons activated by both pellet procurement and dPAG stimulation were not included in the 'stim cell' category. Additionally, we have included a graphic in the revised manuscript (Fig. S3C) that presents the distribution of response latencies of BLA neurons to dPAG stimulation.
(3) To strengthen the claim that it is a BLA-PVT-PAG circuit that carries information about predatory threat, a new experiment using CTB and cFos could be used to demonstrate that PAG neurons that project to PVT are recruited during the robot exposure.
Our study primarily aimed to explore the transmission of threat signals between the dPAG and BLA. We acknowledge that our evidence for the PVT’s intermediary role, derived from CTB injections in the BLA and subsequent CTB+cFos co-labeling analysis in the PVT (Fig. 4G and 4H), is limited. Accordingly, we have moderated the emphasis on the PVT’s involvement in both the abstract and introduction. We now present the PVT’s role as a promising direction for future research in the discussion section of our revised manuscript.
(4) In Fig 2, the authors' interpretation is that photostimulation of PAG neurons elicits fleeing responses in the rats. However, there is a vast literature demonstrating that the PAG is also involved in nociception. Although this is recognized by the authors in the first part of the introduction and briefly described in the discussion, the authors should more explicitly explain that PAG stimulation produces analgesia and thus is unlikely to underlie the escaping responses observed. This may not be intuitive for a broader audience.
We appreciate the reviewer's insightful suggestion to elaborate on the PAG involvement in nociception and analgesia, as supported by the literature. While our initial manuscript acknowledged these functions, we have now expanded our discussion to address the PAG’s multifaceted roles (pg. 12): “As mentioned in the introduction, the dPAG is recognized as part of the ascending nociceptive pathway to the BLA (De Oca et al. 1998, Gross and Canteras 2012, Herry and Johansen 2014, Kim, Rison, and Fanselow 1993, Ressler and Maren 2019, Walker and Davis 1997). The dPAG is also implicated in non-opioid analgesia (e.g., Bagley and Ingram 2020, Cannon et al. 1982, Fields 2000). However, it is essential to emphasize that, despite its roles in pain modulation, the primary behavior observed in dPAG-stimulated, naive rats foraging for food in an open arena was goal-directed escape to the safe nest, underscoring the dPAG’s critical function in survival behaviors.” Note that this aligns with human studies on PAG stimulation (e.g., Carrive and Morgan 2012, Magierek et al. 2003), particularly those by Amano et al. (Amano et al. 1982), which reported patients feeling an urge to escape, similar to being chased, upon PAG stimulation.
(5) To truly demonstrate the functional links between the PAG and BLA, more experiments are needed. For example, one could record from BLA neurons during the robot surge while performing optogenetic inhibition of the PAG neurons. There is also no evidence that activity in the indirect pathway that connects the PAG to the BLA is indispensable for the expression of defensive responses towards the robot (e.g., causality tests using chemogenetic or optogenetic inactivation).
We agree that incorporating optogenetic inhibition of PAG neurons while simultaneously recording from BLA neurons during a robot surge would strengthen the evidence for the functional connectivity between the PAG and BLA. Such an experiment would necessitate the transfection and photoinhibition of a wide array of dPAG neurons responsive to predatory threats. This procedure is technically more viable in transgenic mouse models, given their suitability for genetic manipulation. In light of this, and in response to the suggestions in the Joint Public Review, we have revised the abstract, introduction, and discussion to offer a more cautious interpretation of our findings. This revision reflects a careful consideration of both the evidence and the limitations inherent in our study (pg. 13): “While our findings demonstrate that opto-stimulation of the dPAG is sufficient to trigger both fleeing behavior and increased BLA activity, we have not established that the dPAG is necessary for the BLA’s response to predatory threats. To establish causality, it is essential to conduct experiments such as optogenetic inhibition to determine whether the dPAG is indispensable for activating BLA neurons and initiating escape behavior in the face of threats. The complexity of targeting the dPAG, which includes its dorsomedial, dorsolateral, lateral, and ventrolateral subdivisions (e.g., Bandler, Carrive, and Zhang 1991, Bandler and Keay 1996, Carrive 1993), suggests the need for future studies using transgenic mouse models. Should inactivation of the dPAG negate the BLA's response to predatory threats, it would underscore the dPAG's central role in this defensive mechanism. Conversely, if BLA responses remain unaffected by dPAG inactivation, this could indicate the existence of multiple pathways for antipredatory defense mechanisms.”
(6) The manuscript lacks information about the number of rats and trials that were used across the experiments (e.g. Fig 2G-J). In some occasions, the authors start the experiments with a specific number of animals and then reduce the N by half without providing a rationale (e.g. Fig. 3). Equally confusing is the experimental timeline. For example: a) Were the pre-robot, robot, and post-robot sessions always performed within the same day? b) It was described that microdrivable arrays were used, but did the same rats experienced the robot test more than one time? c) How many bins were used for normalization during the Z-score calculation and when were the data binned at 100 ms versus 1 s? d) How many trials were used for each analysis? For example, to identify robot cells, did the authors establish a minimum number of trials per animal to calculate the peristimulus time histograms? Having a significant number of trials is critical to make sure that the observed neuronal responses are replicable across the trials. e) How was the neuronal activity related to "pellet retrieval" aligned during robot sessions? Was the activity aligned with the moment in which the rat touches the pellet or when the animal returns to the nest with the pellet? f) How did the authors control for trials in which the rat consumed the pellets in the same local vs. those in which they returned to the nest to eat it? All these points are extremely important for future replicability.
We apologize for any confusion caused by the initial lack of detail in our experimental procedures. The revised manuscript has been updated with comprehensive methodological details:
(i) The study involved thirteen rats (ChR2, n = 9; EYFP, n = 4), subjected to dPAG stimulation using fixed light parameters (473 nm, 20 Hz, 10-ms pulse width, 2 s duration) during Long and Short pellet distance trials (refer to Fig. 2E-G). The stimulation intensity was adjusted to each animal's response (fleeing behavior), ranging from 1-3 mW. Additional testing occurred over multiple days, with incremental adjustments to stimulation parameters (intensity, frequency, duration) after confirming normal baseline foraging behavior (Fig. 2H-J, at x = 0). These details are now clearly depicted in the manuscript.
(ii) The primary objective was to investigate BLA neuron responses to dPAG opto-stimulation. Six rats were initially tested, with three later assessed for their reactions to dPAG stimulation in the presence of an actual predator, to gauge behavioral effects.
(iii) Regarding the experimental timeline:
a) Pre-robot, robot, and post-robot sessions were conducted successively on the same day.
b) Sessions with the robot predator were repeated until habituation occurred or when unit recordings were deemed invalid due to microdrive limitations or the absence of unit detection. Throughout these sessions, the success rate for pellet retrieval remained consistently low. Specifically, the mean success rate for the dPAG recordings was 2.803% + 1.311. For the BLA recordings, animals did not succeed in retrieving pellets during any of the robot trials. To provide a more detailed account of the methodology, the manuscript has been updated to include the number of recording days and the units recorded in the "Behavioral Procedures" section.
c) As described in Materials and Methods, unit recording data were binned at 0.1-s intervals and normalized against a 5-s pre-event baseline (50 bins). For statistical analyses in Figure 1F’s rightmost column, 1-s bins were used to simplify post-hoc analysis corrections.
d) Each recording session consisted of 5-15 trials. Trials were excluded if rats attempted to procure the pellet within 10 s post-dPAG stimulation or robot activation, ensuring accurate characterization of unit responsiveness. Consequently, the number of trials varied among subjects.
e) Pellet retrieval was indicated by the animal entering a designated zone 19 cm from the pellet, driven by hunger.
f) Animals were trained to retrieve pellets and return to their nest for consumption prior to robot testing sessions, as elaborated in the “Baseline foraging” section.
(7) In the abstract, the authors mention that predictive cues are ambiguous during naturalistic predatory threats, but it is not clear what do they mean by ambiguous. In addition, in the introduction section, the authors describe that the present study will investigate how the dPAG and BLA communicate threat signals. However, the author should clarify right in the beginning that these two regions are not monosynaptically connected with each other and cite the proper references.
The abstract’s original sentence, “…where predictive cues are ambiguous and do not afford reiterative trial-and-error learning…” has been refined to “…characterized by less explicit cues and the absence of reiterative trial-and-error learning events …” This adjustment more accurately reflects that cues in natural settings often lack the clear and consistent quality of those in controlled experimental settings, which is necessary for the straightforward process of trial-and-error learning.
Regarding the dPAG and BLA connectivity, the revised introduction (pg. 5) now states: “Considering the lack of direct monosynaptic projections between dPAG and BLA neurons (Vianna and Brandao 2003, McNally, Johansen, and Blair 2011, Cameron et al. 1995), we utilized anterograde and retrograde tracers in the dPAG and BLA, respectively. This was complemented by c-Fos expression analysis following exposure to predatory threats. Our anatomical findings suggest that the paraventricular nucleus of the thalamus (PVT) may be part of a network that conveys predatory threat information from the dPAG to the BLA.”
(8) In the introduction section, the authors should clarify that the US information is conveyed from the PAG to BLA via the lateral thalamus (posterior intralaminar nucleus, medial geniculate nucleus) or dorsal midline thalamus (paraventricular nucleus of the thalamus). The statement regarding how "the PAG functions as part of the ascending pain transmission pathway, providing footshock US information to the BLA" is misleading because the PAG does not send monosynaptic projections directly to the BLA.
The revised text (pg. 3) now reads: “…suggest that the dPAG is part of the ascending US pain transmission pathway to the BLA, the presumed site for CS-US association formation (De Oca et al. 1998, Gross and Canteras 2012, Herry and Johansen 2014, Kim, Rison, and Fanselow 1993, Ressler and Maren 2019, Walker and Davis 1997). This pathway is thought to be mediated through the lateral and dorsal-midline thalamus regions, including the posterior intralaminar nucleus and paraventricular nucleus of the thalamus (Krout and Loewy, 2000; McNally, Johansen, and Blair, 2011; Yeh, Ozawa, and Johansen, 2021; but see Brunzell and Kim, 2001).”
(9) The author's assumption that threat information flows from the PAG to the BLA, rather than BLA to PAG, based on electrical stimulation and lesion experiments performed in previous studies is problematic for at least three reasons: a) Electrical stimulation can activate fibers of passage as well as presynaptic neurons antidromically. b) The lesion approach may not have targeted 100% of the neurons in PAG, which extends anatomically along the antero-posterior axis of the midbrain for several millimeters in rats. This observation also disagrees with more recent studies using optogenetics and imaging tools demonstrating that the PAG is the downstream target of the BLA-CeA pathway. c) The authors cited prior reports describing the role of the amygdala-PAG pathway in dampening the US response and providing a negative signal to the PAG. However, a series of previous studies demonstrating that the PAG serves as the downstream target of the central nucleus of the amygdala for the expression of defensive response are completely ignored by the authors. Here are just some examples: Massi et al, 2023, PMID: 36652513; Tovote et al 2016, PMID: 27279213; Penzo et al, 2014 PMID: 24523533).
We recognize the complexities in interpreting findings from electrical stimulation and lesion studies. Our prior work (Kim et al. 2013) supports the conclusion that predatory threat information directionally flows from the dPAG to the BLA, as evidenced by distinct behavioral outcomes from experimental manipulations of dPAG and BLA. Specifically, dPAG stimulation-induced fleeing behavior was blocked by BLA lesions (as well as muscimol inactivation), whereas BLA stimulation-induced fleeing was unaffected by dPAG or combined dPAG+vPAG lesions (refer to Fig. 5A), suggesting a flow from dPAG to BLA. Our manuscript further clarifies that dPAG optostimulation results confirmed that escape behavior in foraging rats, induce by dPAG electrical stimulation (Kim et al. 2013), was activated by intrinsic dPAG neurons rather than by fibers of passage or current spread to other brain regions.
Furthermore, the PAG’s anatomical and functional diversity, with distinct segments along its longitudinal axis associated with different defensive behaviors, reinforces our conclusions. The dPAG is implicated in flight responses, while the vPAG is associated with freezing behavior (e.g., Bandler and Shipley 1994, Kim, Rison, and Fanselow 1993, Lefler, Campagner, and Branco 2020, Morgan, Whitney, and Gold 1998). The critiques' referenced studies primarily focus on the BLA-CeA-vPAG circuit's role in freezing during Pavlovian fear conditioning, contrasting with our emphasis on the dPAG-PVT-BLA circuit and its mediation in escape behavior in response to naturalistic predatory threats.
We also note that different invasive procedures can yield varying behavioral outcomes. For example, both acute (e.g., optogenetic and muscimol inactivation) and chronic (e.g., surgical ablation) manipulations within the same brain circuit have shown diverse effects across species (Otchy et al. 2015). Moreover, optogenetics comes with its own set of conceptual and technical challenges (Adamantidis et al. 2015), including the difficulty of targeting, quantifying and photo-inhibiting 100% of PAG neurons. Despite the limitations of each technique, our collective evidence from lesions, inactivation, electrical stimulation (Kim et al. 2013), optostimulation, and single-unit recordings (the present study) supports the premise that the dPAG acts upstream of the BLA in processing predatory threat information.
(10) In the discussion, the authors suggest that the PVT may be the interface between the PAG and the BLA for the expression of antipredatory defensive behavior during their foraging vs. robot test, but previous studies looking at the role of PVT in antipredator defensive behavior and/or approach-avoidance conflict tasks are not cited and discussed in the manuscript (Engelke et al, 2021, PMID: 33947849; Choi et al 2019, PMID: 30979815; Choi and McNally 2017, PMID: 28193686).
We thank the reviewer for pointing out these pivotal studies, which we have carefully reviewed and integrated into the revised manuscript (pg. 14): “These results, in conjunction with previous research on the roles of the dPAG, PVT, and BLA in producing flight behaviors in naïve rats (Choi and Kim 2010, Daviu et al. 2020, Deng, Xiao, and Wang 2016, Kim et al. 2013, Kim et al. 2018, Kong et al. 2021, Ma et al. 2021, Reis et al. 2021), the anterior PVT’s involvement in cat odor-induced avoidance behavior (Engelke et al. 2021), and the PVT’s regulation of behaviors motivated by both appetitive and aversive stimuli (Choi and McNally 2017, Choi et al. 2019), suggest the involvement of the dPAGàPVTàBLA pathways in antipredatory defensive mechanisms, particularly as rats leave the safety of the nest to forage in an open arena (Figure 4I) (Reis et al. 2023).”
(11) The authors use the expression "looming robot predator" in many cases throughout the manuscript. However, it is unclear whether the defensive responses observed in the rats are elicited by the looming stimulus produced by the movement of the robot towards the rats. The authors describe that rats do not respond to a stationary robot, but would the sound produced by the movement of the robot elicit defensive responses? Would non-approaching lateral or dorsoventral movements (not associated with looming) be sufficient to induce defensive behavior in the rats? There is a vast literature in the field about defensive behaviors induced by looming stimuli. The authors should empirically demonstrate that the escaping responses induced by the robot are mediated by looming or refrain to use the looming terminology to avoid confusion.
Our use of "looming robot predator" is based on empirical evidence from a prior parametric study, which identified the forward, or 'looming,' motion of the Robogator as the key stimulus eliciting a flight response in rats (Kim, Choi, and Lee 2016). This reaction significantly decreased when the robot moved backward from the same starting position, producing a similar sound, and was absent when the robot remained stationary. This suggests that neither sound alone nor the mere presence of a novel object provokes goal-directed escape behavior (Kong et al. 2021). This aligns with studies indicating that simulated looming stimuli, like an expanding disk, induce flight or freezing responses in mice (De Franceschi et al. 2016, Yilmaz and Meister 2013).
It should be noted that the 2013 study by Yilmaz & Meister (Yilmaz and Meister 2013) on the looming disk paradigm showed that not all mice responded to the stimuli (e.g., Figs. 2A and 3A), with those that did exhibiting rapid habituation by the second exposure. This contrasts with our predatory robot paradigm (Choi and Kim 2010), where all rats consistently fled from the looming robotic predator across multiple trials, underscoring the critical role of looming motion in simulating predator attacks that trigger flight behavior in rats.
Thus, the term "looming" accurately captures the nature of the robot's movement and its effect on eliciting defensive responses in rats. Nonetheless, should the editors agree with the reviewer's suggestion to minimize potential confusion, we are willing to substitute "looming" with "approaching," although we consider the terms to be synonymous in the context of our study.
(12) If the authors are citing the Rescorla-Wagner model, they should include at least one additional sentence to explain it, as many people in the field are not familiar with this model.
In response to the request for clarification on the Rescorla-Wagner model, we have added an explanatory sentence (pg. 4): “Fundamentally, the negative feedback circuit between the amygdala and the dPAG serves as a biological implementation of the Rescorla–Wagner (1972) model, a foundational theory of associative learning that emphasizes the importance of prediction errors in reinforcement (i.e., US), as applied to FC (Fanselow 1998).”
(13) The authors need to include the normality test used to determine whether a parametric or non-parametric statistical analysis was the most appropriate test for each experiment.
We have included the outcomes of the normality tests, detailed in Table S1.
(14) In Fig. 1F, the authors show a representative PAG neuron with peristimulus-time histogram and rasters reaching frequencies higher than 100 Hz and sustained firing rates of >50 Hz following robot activation. The authors should include a firing rate analysis (e.g., average firing rate and maximum firing rate before and after robot activation) of the 22 robot-responsive PAG neurons recorded during the session to clarify whether this high firing rate, which is atypical in other brain regions, is commonly observed in the PAG. Showing the isolated waveforms of some representative neurons would help to clarify whether the activity is being recorded from a single-isolated unit instead of multiple units within the same channel.
In response to the critique, we have expanded our analysis to include both average and maximum firing rates before and after robot activation for the 22 robot-responsive PAG neurons. This detailed firing rate analysis, illustrating their distribution, has been incorporated into the revised manuscript (refer to Figure S1C and S1D). Furthermore, to alleviate concerns regarding the identification of single-unit activity versus potential multi-unit recordings, we have included peri-event raster plots and waveforms for two additional representative neurons in Figure 1F.
(15) In Figure 2, the authors should indicate when the recordings are performed on anesthetized vs. freely-moving awake animals.
In the original manuscript, we specified that the optrode recordings depicted in Figure 2B were conducted on anesthetized rats. To enhance clarity and directly address the critique, we have now clearly indicated this condition in Figure 2A as well.
(16) The optogenetic stimulation parameters used in Fig 2H indicate that 0.5 mW was sufficient to induce behavioral changes. This is surprising because most optogenetic experiments in the field use much higher intensities (> 5mW). If much lower intensities are sufficient to drive PAG-mediated behaviors, this may be a very important observation that should be conveyed to the field. I recommend the reviewers clarify if they in fact used 0.5 mW and then discuss that the laser intensity used in the experiments was 10X lower than that required for other brain regions
In our study, we indeed observed that 0.5 mW of dPAG stimulation increased the latency to procure the pellet without completely preventing the action. Notably, at 1 mW, more than half of the animals (n = 5/9 rats; Fig. 2H) and at 3 mW, all rats (9/9) failed to procure the pellet and fled from the foraging area to the nest (Fig. 2G). These results indicate that even lower intensities were sufficient to elicit behavioral changes through dPAG stimulation in a large foraging arena, highlighting the dPAG's sensitivity to optogenetic manipulation. This finding is consistent with our earlier research on dPAG electrical stimulation, which required significantly lower intensities to provoke defensive behaviors compared to the BLA. Specifically, the stimulation intensity needed for aversive behavior in the dPAG was substantially lower (dPAG: 65.0 ± 6.85 µA) than for the BLA (BLA: 275.0 ± 24.44 µA) (Kim et al. 2013). Furthermore, Deng et al. (Deng, Xiao, and Wang 2016) showed that 1 mW of blue light could elicit a 60% freezing response, with 2 mW triggering flight behavior within a latency of 0.6 seconds.
(17) In Fig 2 G-J, how many animals are being used per group and how was the sequence of the experiments performed? This is very important for replicability.
A total of three rats were utilized for the robot testing experiments depicted in Fig. 2 G-J. The experimental sequence for these animals consisted of successive pre-stimulation, stimulation, post-stimulation, and robot sessions. We have updated the manuscript to include this information.
(18) For the photostimulation of PAG neurons in Figs. 2 and 3, the authors need to clarify if the same parameters of laser stimulation used during the anesthetized recordings were also used during the behavioral tests. Also, the wavelength corresponding to the blue laser should be 473 nm instead of 437 nm.
We thank the reviewer for identifying the error. We confirm that the opto-stimulation parameters (473 nm, 10-ms pulse width, 2 s duration) were consistently applied across both anesthetized recordings and behavioral tests. This consistency has been explicitly stated in the revised manuscript to ensure clarity regarding our experimental approach.
(19) In Fig. 3I, how was the representative trials selected? Instead of picking up the most representative trials, the authors should demonstrate the response of the cell during the entire session.
In response to the critique, we clarify that the color-coded PETH shown in Fig. 3I represents averaged BLA activity across a comprehensive set of trials. This includes 8 pre-stimulation, 10 stimulation, and 8 post-stimulation trials for the robot-activated sessions, with a similar distribution for non-stimulated sessions. This approach was chosen to provide a representative overview of the cell's response throughout the entire session. To address the request for more detailed data, we have added traditional PETHs to the revised manuscript (see Fig. S3H), which depict the cell's response across all trials.
(20) Fig 4 D should demonstrate a colabeling between the anterograde PAG fibers in the PVT and the retrogradely labeled neurons from BLA instead of PAG fibers only.
We wish to clarify that Fig. 4D is intended to show the distribution of dPAG terminals within the midline thalamic nuclei, as noted in prior research (Krout and Loewy 2000). Although dPAG terminals are distributed throughout the midline thalamus, our observations have specifically highlighted a notable increase in c-Fos expression within the paraventricular nucleus of the thalamus (PVT) in rats subjected to the robotic predator stimulus, in contrast to those in the foraging-only control condition (Fig. 4E). Addressing the reviewer's point, we direct attention to Fig. 4G, which includes images labeled "Robot-experienced" and "Merge." This figure demonstrates a subset of PVT neurons that were retrogradely labeled with CTB injected into the BLA, anterogradely labeled with AAV injected into the dPAG, and activated (as indicated by c-Fos expression) in response to the robotic predator. This provides specific colabeling evidence between anterograde PAG fibers in the PVT and retrogradely labeled neurons from the BLA, directly addressing the critique.
(21) The resolution of the cFos images is very low and makes it hard to appreciate.
We have updated Figs. 4F and 4G with high-resolution versions to ensure the details are more clearly visible. Furthermore, should there be a need for even greater clarity, we are prepared to supply the images as TIFF files, which are known for preserving high image quality.
Reviewer 2:
(1) The text is clearly written, and I appreciated the inclusion of interesting citations, such as the one about paintings by cavemen. The authors also do a good job of discussing the underlying theoretical framework and the figures are easy to understand. Although the topic is very interesting, the amount of novel work is somewhat low. Figure 1 shows that dPAG cells are activated by the predator, and this has been shown by many prior reports. Similarly, Figure 2 shows that dPAG activation creates defensive responses, and this too has been shown by many prior reports.
We appreciate the reviewer’s positive remarks. We acknowledge the rich body of research documenting dPAG neuronal activation by various predator cues such as odors (e.g., fox urine) (Lu et al. 2023), and scenarios involving anesthetized or spontaneously moving rat/cat predators, either physically partitioned or harness-restrained (Bindi et al. 2022, Deng, Xiao, and Wang 2016, Esteban Masferrer et al. 2020). Nevertheless, our study distinguishes itself by examining dPAG neuronal responses to a robotic predator, uniquely designed to replicate consistent looming motions across multiple trials and subjects within an environment that simulates natural foraging conditions, inclusive of a safe nest (cf. Choi and Kim, 2010). This approach allowed us to not only reveal the immediate activation of dPAG neurons in response to a rapidly approaching predator but also to explore the consequent fleeing behavior towards safety, thereby providing new insights into the dPAG's role in mediating goal-directed defensive responses in a more ecologically-relevant setting. Furthermore, our investigation extends beyond these findings to assess the impact of dPAG activation on BLA neuronal responses and their functional connectivity during predator-prey interactions, offering a fresh perspective on the neural circuits that support survival behaviors in animals when confronted with naturalistic threats.
(2) The results in Figure 3 are novel and interesting, but the characterization of BLA activity is incomplete. For example, what are the percentages of BLA cells that are inhibited or activated by all major behaviors observed? These behaviors include approach to pellet, escape from robot, freezing, stretch-attend postures, etc. These same analyses should also be added to dPAG activity in Figure 1. How does BLA single cell encoding of these behaviors relate to their responsivity to dPAG stimulation? And, finally, it is unclear what is the significance of BLA correlated synchronous firing. Is the animal more or less likely to be performing certain behaviors when correlated BLA firing occurs?
Our analysis, as presented in Figs. 3I, 3K, and S3D-F, selectively focused on BLA cell responses during distinct behaviors such as approaching a pellet and escaping from the robot. These behaviors were selected because their precise temporal markers allow for accurate correlation with BLA cell activity, building on the findings of our previous research (Kim et al. 2018, Kong et al. 2021).
The robot's motion, programmed to advance a fixed distance before retreating to its starting position, is designed to repeatedly elicit foraging, thus facilitating analysis of neural changes during conflict situations involving food approach and predator avoidance. However, this also leads to the rapid diminution of freezing and stretch-attend postures inside the nest as animals quickly adapt to the robot's movement pattern, rendering a time-stamped analysis of these behaviors unfeasible under our experimental conditions. While the inclusion of these behaviors in our analysis would be insightful, especially in extended interaction scenarios where the robot advances to the nest opening and remains before returning in a less predictable manner, such conditions would likely reduce foraging behavior due to increased fear, deviating from our study's primary objective of elucidating the interactions between the dorsal periaqueductal gray (dPAG) and the basolateral amygdala (BLA) functions.
Regarding the significance of BLA correlated synchronous firing, our findings, particularly in Figures 3M-O and S4, demonstrate significant synchronous activity among BLA neuronal pairs during encounters with the robot, as opposed to pre-stim, stim, and post-stim sessions. This synchrony is notably prominent among neurons responsive to dPAG stimulation, indicating that BLA neurons involved in processing dPAG signals may play a crucial role in enhancing BLA network coherence to effectively manage predatory threat information (pg. 13).
(3) In Figure 4, the authors identify the PVT as a potential region that can mediate dPAG to BLA communication via anatomical tracing. However, functional assays are missing. For example, if the PVT is inhibited chemogenetically, does this result in a smaller number of BLA cells that are activated by dPAG stimulation? Does activation of the dPAG-PVT or the PVT-BLA projections cause defensive behaviors? Functionally showing that the dPAG-PVT-BLA circuit controls defensive actions would be a major advance in the field and would greatly enhance the significance of this paper. It would also provide an anatomical substrate to support the view that the BLA is downstream of the dPAG, which was first demonstrated by the authors in their elegant 2013 PNAS paper.
We appreciate the reviewer’s constructive critique and valuable suggestions on the necessity for functional validation of the dPAG-PVT-BLA circuit's involvement in mediating defensive behaviors. In light of these comments, we have carefully considered and included a discussion on the importance of these proposed experiments as a direction for future research in our manuscript revision (also see response to Reviewer 1’s critique #5).
Our initial work in 2013 (Kim et al. 2013) laid the groundwork for identifying BLA neurons responsive to dPAG stimulation and suggested the PVT as a potential relay in this neural circuit. Recognizing the limitations of our current study, which does not include direct functional assays, we have adjusted our manuscript to convey the speculative aspect of the dPAG-PVT-BLA circuit’s role more accurately. Moreover, we have enriched our discussion by citing relevant studies that lend support to our proposed circuit mechanism. These references serve to place our findings within the broader context of existing research and highlight the imperative for subsequent studies to empirically confirm the functional significance of the dPAG-PVT-BLA pathway in driving defensive behaviors.
Reviewer 3:
(1) The Introduction refers to a negative feedback amygdala-dPAG from a study of the Johansen group, but in this case, the authors were referring to the ventrolateral and not the dorsal PAG.
We thank the reviewer for pointing out the need to distinguish between the dPAG and vPAG regions in our introduction. While Johansen et al. (2010) investigated the roles of PAG (including both dPAG and vPAG regions; see their Supplementary Figs. 4, 5, and 10), the differentiation between their specific contributions to the amygdala's negative feedback mechanism was not explicitly detailed in their initial publication. This distinction was further elaborated upon in later work by the same group (Yeh, Ozawa, and Johansen 2021), which specifically illuminated the dPAG's role in conditioned fear memory formation and its neural pathways to the PVT that influence fear learning. To reflect this nuanced understanding, we have revised our introduction (pg. 3): “In parallel, Johansen et al. (2010) found that pharmacological inhibition of the PAG, encompassing both dPAG and vPAG regions, diminishes the behavioral and neural responses in the amygdala elicited by periorbital shock US, thereby impairing the acquisition of auditory FC.”
(2) In the experiments recording dPAG in response to the predator threat, the authors mentioned cells activated by the predator threat, referred to as "robot cells." Were these cells inhibited in response to threat?
In the Result and Materials and Methods sections, we report that 23.4% (22 out of 94) of dPAG neurons, termed “robot cells,” showed a significant increase in firing rates (z > 3) within a latency of less than 500 ms during exposure to the looming robot threat, but not during the pre- and post-robot sessions. These cells are highlighted in Figures 1E-G. In contrast, we identified only a single unit exhibiting a decrease in activity (z-score < -3) in response to the robot threat. Given the overwhelming prevalence of cells with excitatory responses to the threat, our discussions and analyses have primarily centered on these excited cells. Nevertheless, to ensure a full depiction of our observations, we have included data on the inhibited unit in the revised manuscript, specifically in Figure S1E.
(3) The authors claim that tetrodes were implanted in the dorsal PAG; however, the electrodes' tips shown in the figures are positioned more ventrally in the lateral PAG (see Figures 1B, S5A).
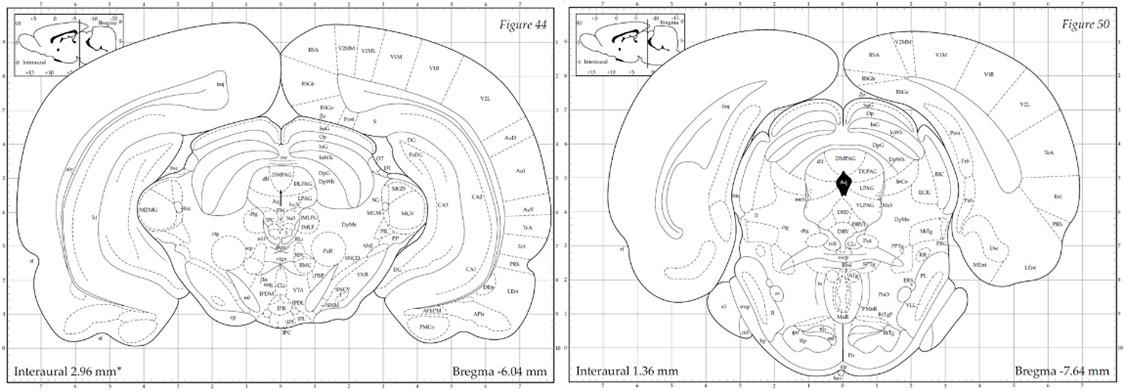
The PAG is anatomically organized into dorsomedial (dmPAG), dorsolateral (dlPAG), lateral (lPAG), and ventrolateral (vlPAG) columns along the rostro-caudal axis of the aqueduct. The designation "dorsal PAG" (dPAG) traditionally encompasses the dmPAG, dlPAG, and lPAG regions, a classification supported by extensive track-tracing, neurochemical, and immunohistochemical evidence (e.g., (Bandler, Carrive, and Zhang 1991, Bandler and Keay 1996, Carrive 1993)). As Bandler and Shipley (Bandler and Shipley 1994) summarized, “These findings suggest that what has been traditionally called the 'dorsal PAG' (a collective term for regions dorsal and lateral to the aqueduct), consists of three anatomically distinct longitudinal columns: dorsomedial and lateral columns…and a dorsolateral column…" Similarly, Schenberg et al. (Schenberg et al. 2005) clarified in their review that, “According to this parcellation...the defensive behaviors (freezing, flight or fight) and aversion-related responses (switch-off behavior) were ascribed to the DMPAG, DLPAG, and LPAG (usually named the ‘dorsal’ PAG).” In our study, electrode placements were strictly within these specified dPAG regions. The electrode tip locations depicted in Figures 1B and S5A correspond with the -6.04 mm template (left panel below) from Paxinos & Watson’s atlas (Paxinos and Watson 1998), situated anteriorly to the emergence of the vlPAG (right panel below). To enhance clarification in our manuscript, we provide a detailed definition of the dPAG that includes the dmPAG, dlPAG, and lPAG, and support our electrode placement rationale with references to established literature (pg. 5).
Author response image 1.
(4) It would be nice to include a series of observations applying inhibitory tools (i.e., optogenetic photo inhibition) in the dPAG and BLA and see how they affect the behavioral responses in the 'approach food-avoid predator' paradigm. Moreover, it would be interesting to explore how inhibiting the dPAG to PVT pathway influences the flee response during the robot surge.
We appreciate the suggestion to explore the effects of optogenetic inhibition in the dPAG and BLA on behavioral responses within the 'approach food-avoid predator' paradigm, as well as the potential impact of inhibiting the dPAG to PVT pathway on flee responses during robot surge incidents. As mentioned in our response to Reviewer 1’s critique #5, the application of optogenetic inhibition necessitates transfecting, quantifying, and photoinhibiting a comprehensive set of dPAG neurons activated by predatory threats. This approach is more viable in future studies that can leverage transgenic mouse models for their genetic tractability. Following the Joint Public Review’s recommendations, we have revised our manuscript to ensure a more measured interpretation of our data, carefully balancing the evidence from tracer studies against the limitations of our current methodology.
Furthermore, referencing Reviewer 1’s critique #9, it is important to consider that various invasive techniques can yield different behavioral outcomes. For instance, research by Olveczky and colleagues (Otchy et al. 2015) demonstrated that acute manipulations (i.e., optogenetic and muscimol inactivation) and chronic surgical ablation of the same brain circuit can produce distinct effects in rats and finches. Despite these methodological constraints, our collective results from lesion, inactivation, electrical stimulation (Kim et al. 2013), optostimulation, and single-unit recording (present) studies cohesively suggest that the dPAG functions upstream of the BLA in processing predatory threat signals.
(5) The authors should also examine whether 'synaptic' appositions exist between the anterogradely labeled terminals from the dPAG and the double labeled CTB and cFOS neurons in the PVT.
We appreciate the suggestion to investigate the presence of synaptic appositions, which could potentially offer valuable insights into the synaptic connections and functional interactions within this neural circuit. However, due to the specialized nature of electron microscopy required for these examinations and the extensive resources it entails, this line of inquiry falls beyond the scope of our current study. We hope to address this aspect in future studies, where we can dedicate the necessary resources and expertise to conducting these intricate analyses.
(6) It is odd to see the projection fields shown in Fig. 4D, where the projection to the PVT looks much sparser compared to other targets in the thalamus and hypothalamus. If the projection to the PVT has such an important function, why does it seem so weak? This should be discussed. Also, because the projection to the PVT seems sparse, the authors should consider alternative paths like the one involving the cuneiform nucleus. The cuneiform nucleus is an important region responding to looming shadows with strong bidirectional links to the dorsolateral periaqueductal gray, providing strong projections to the rostral PVT.
The perceived scarcity of the dPAG-PVT pathway might not reflect its functional significance accurately. The PVT's small size could make its projections appear less dense in broad anatomical studies. To address this, we have updated Figure 4D with a high-resolution image that offers a detailed view of the PVT region. This enhancement (refer to the updated Fig. 4, bottom) more accurately depicts the projection density within the PVT. It is also critical to consider that the functional impact of neural pathways is not solely dependent on the quantity of projecting neurons. For instance, work by Deisseroth and colleagues (Rajasethupathy et al. 2015) has shown that even relatively sparse monosynaptic projections from the anterior cingulate cortex to the hippocampus can exert significant effects on neural circuit dynamics. Additionally, we have expanded our discussion to consider the potential roles of other circuits, such as the cuneiform nucleus, in driving the behavioral responses observed in our study (pg. 15): “Given the recent significance attributed to the superior colliculus in detecting innate visual threats (Lischinsky and Lin 2019, Wei et al. 2015, Zhou et al. 2019) and the cuneiform nucleus in the directed flight behavior of mice (Bindi et al. 2023, Tsang et al. 2023), further exploration into the communication between these structures and the dPAG-BLA circuitry is warranted.”
(7) Finally, in the Discussion, it would be nice to comment on how the BLA mediates flee responses. Which pathways are likely involved?
This excellent suggestion has been incorporated in the discussion (pg. 15): “Future studies will also need to delineate the downstream pathways emanating from the BLA that orchestrate goal-directed flight responses to external predatory threats as well as internal stimulations from the dPAG/BLA circuit. Potential key structures include the dorsal/posterior striatum, which has been associated with avoidance behaviors in response to airpuff in head-fixed mice (Menegas et al. 2018) and flight reactions triggered by auditory looming cues (Li et al. 2021). Additionally, the ventromedial hypothalamus (VMH) has been implicated in flight behaviors in mice, evidenced by responses to the presence of a rat predator (Silva et al. 2013) and upon optogenetic activation of VMH Steroidogenic factor 1 (Kunwar et al. 2015) or the VMH-anterior hypothalamic nucleus pathway (Wang, Chen, and Lin 2015). Investigating the indispensable role of these structures in flight behavior could involve lesion or inactivation studies. Such interventions are anticipated to inhibit flight behaviors elicited by amygdala stimulation and predatory threats, confirming their critical involvement. Conversely, activating these structures in subjects with an inactivated or lesioned amygdala, which would typically inhibit fear responses to external threats (Choi and Kim 2010), is expected to induce fleeing behavior, further elucidating their functional significance.”
Adamantidis, A., S. Arber, J. S. Bains, E. Bamberg, A. Bonci, G. Buzsaki, J. A. Cardin, R. M. Costa, Y. Dan, Y. Goda, A. M. Graybiel, M. Hausser, P. Hegemann, J. R. Huguenard, T. R. Insel, P. H. Janak, D. Johnston, S. A. Josselyn, C. Koch, A. C. Kreitzer, C. Luscher, R. C. Malenka, G. Miesenbock, G. Nagel, B. Roska, M. J. Schnitzer, K. V. Shenoy, I. Soltesz, S. M. Sternson, R. W. Tsien, R. Y. Tsien, G. G. Turrigiano, K. M. Tye, and R. I. Wilson. 2015. "Optogenetics: 10 years after ChR2 in neurons--views from the community." Nat Neurosci 18 (9):1202-12. doi: 10.1038/nn.4106.
Amano, K., T. Tanikawa, H. Kawamura, H. Iseki, M. Notani, H. Kawabatake, T. Shiwaku, T. Suda, H. Demura, and K. Kitamura. 1982. "Endorphins and pain relief. Further observations on electrical stimulation of the lateral part of the periaqueductal gray matter during rostral mesencephalic reticulotomy for pain relief." Appl Neurophysiol 45 (1-2):123-35.
Bagley, E. E., and S. L. Ingram. 2020. "Endogenous opioid peptides in the descending pain modulatory circuit." Neuropharmacology 173:108131. doi: 10.1016/j.neuropharm.2020.108131.
Bandler, R., P. Carrive, and S. P. Zhang. 1991. "Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization." Prog Brain Res 87:269-305. doi: 10.1016/s0079-6123(08)63056-3.
Bandler, R., and K. A. Keay. 1996. "Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression." Prog Brain Res 107:285-300. doi: 10.1016/s0079-6123(08)61871-3.
Bandler, R., and M. T. Shipley. 1994. "Columnar organization in the midbrain periaqueductal gray: modules for emotional expression?" Trends Neurosci 17 (9):379-89. doi: 10.1016/0166-2236(94)90047-7.
Bindi, R. P., C. C. Guimaraes, A. R. de Oliveira, F. F. Melleu, M. A. X. de Lima, M. V. C. Baldo, S. C. Motta, and N. S. Canteras. 2023. "Anatomical and functional study of the cuneiform nucleus: A critical site to organize innate defensive behaviors." Ann N Y Acad Sci 1521 (1):79-95. doi: 10.1111/nyas.14954.
Bindi, R. P., R. G. O. Maia, F. Pibiri, M. V. C. Baldo, S. L. Poulter, C. Lever, and N. S. Canteras. 2022. "Neural correlates of distinct levels of predatory threat in dorsal periaqueductal grey neurons." Eur J Neurosci 55 (6):1504-1518. doi: 10.1111/ejn.15633.
Cameron, A. A., I. A. Khan, K. N. Westlund, and W. D. Willis. 1995. "The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections." J Comp Neurol 351 (4):585-601. doi: 10.1002/cne.903510408.
Cannon, J. T., G. J. Prieto, A. Lee, and J. C. Liebeskind. 1982. "Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat." Brain Res 243 (2):315-21. doi: 10.1016/0006-8993(82)90255-4.
Carrive, P, and M. M. Morgan. 2012. "Periaqueductal Gray." In The Human Nervous System, edited by J. K.; Paxinos Mai, G., 367-400. London: Academic Press.
Carrive, P. 1993. "The periaqueductal gray and defensive behavior: functional representation and neuronal organization." Behav Brain Res 58 (1-2):27-47. doi: 10.1016/0166-4328(93)90088-8.
Choi, E. A., P. Jean-Richard-Dit-Bressel, C. W. G. Clifford, and G. P. McNally. 2019. "Paraventricular Thalamus Controls Behavior during Motivational Conflict." J Neurosci 39 (25):4945-4958. doi: 10.1523/JNEUROSCI.2480-18.2019.
Choi, E. A., and G. P. McNally. 2017. "Paraventricular Thalamus Balances Danger and Reward." J Neurosci 37 (11):3018-3029. doi: 10.1523/JNEUROSCI.3320-16.2017.
Choi, J. S., and J. J. Kim. 2010. "Amygdala regulates risk of predation in rats foraging in a dynamic fear environment." Proc Natl Acad Sci U S A 107 (50):21773-7. doi: 10.1073/pnas.1010079108.
De Franceschi, G., T. Vivattanasarn, A. B. Saleem, and S. G. Solomon. 2016. "Vision Guides Selection of Freeze or Flight Defense Strategies in Mice." Curr Biol 26 (16):2150-4. doi: 10.1016/j.cub.2016.06.006.
De Oca, B. M., J. P. DeCola, S. Maren, and M. S. Fanselow. 1998. "Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses." J Neurosci 18 (9):3426-32. doi: 10.1523/JNEUROSCI.18-09-03426.1998.
Deng, H., X. Xiao, and Z. Wang. 2016. "Periaqueductal Gray Neuronal Activities Underlie Different Aspects of Defensive Behaviors." J Neurosci 36 (29):7580-8. doi: 10.1523/JNEUROSCI.4425-15.2016.
Engelke, D. S., X. O. Zhang, J. J. O'Malley, J. A. Fernandez-Leon, S. Li, G. J. Kirouac, M. Beierlein, and F. H. Do-Monte. 2021. "A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats." Nat Commun 12 (1):2517. doi: 10.1038/s41467-021-22730-y.
Esteban Masferrer, M., B. A. Silva, K. Nomoto, S. Q. Lima, and C. T. Gross. 2020. "Differential Encoding of Predator Fear in the Ventromedial Hypothalamus and Periaqueductal Grey." J Neurosci 40 (48):9283-9292. doi: 10.1523/JNEUROSCI.0761-18.2020.
Fanselow, M. S. 1998. "Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation." Neuron 20 (4):625-7. doi: 10.1016/s0896-6273(00)81002-8.
Fields, H. L. 2000. "Pain modulation: expectation, opioid analgesia and virtual pain." Prog Brain Res 122:245-53. doi: 10.1016/s0079-6123(08)62143-3.
Gross, C. T., and N. S. Canteras. 2012. "The many paths to fear." Nat Rev Neurosci 13 (9):651-8. doi: 10.1038/nrn3301.
Herry, C., and J. P. Johansen. 2014. "Encoding of fear learning and memory in distributed neuronal circuits." Nat Neurosci 17 (12):1644-54. doi: 10.1038/nn.3869.
Kim, E. J., O. Horovitz, B. A. Pellman, L. M. Tan, Q. Li, G. Richter-Levin, and J. J. Kim. 2013. "Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats." Proc Natl Acad Sci U S A 110 (36):14795-800. doi: 10.1073/pnas.1310845110.
Kim, E. J., M. S. Kong, S. G. Park, S. J. Y. Mizumori, J. Cho, and J. J. Kim. 2018. "Dynamic coding of predatory information between the prelimbic cortex and lateral amygdala in foraging rats." Sci Adv 4 (4):eaar7328. doi: 10.1126/sciadv.aar7328.
Kim, J. J., J. S. Choi, and H. J. Lee. 2016. "Foraging in the face of fear: Novel strategies for evaluating amygdala functions in rats." In Living without an amygdala, edited by D. G. Amaral and R. Adolphs, 129-148. The Guilford Press.
Kim, J. J., R. A. Rison, and M. S. Fanselow. 1993. "Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear." Behav Neurosci 107 (6):1093-8. doi: 10.1037//0735-7044.107.6.1093.
Kong, M. S., E. J. Kim, S. Park, L. S. Zweifel, Y. Huh, J. Cho, and J. J. Kim. 2021. "'Fearful-place' coding in the amygdala-hippocampal network." Elife 10. doi: 10.7554/eLife.72040.
Krout, K. E., and A. D. Loewy. 2000. "Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat." J Comp Neurol 424 (1):111-41. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3.
Kunwar, P. S., M. Zelikowsky, R. Remedios, H. Cai, M. Yilmaz, M. Meister, and D. J. Anderson. 2015. "Ventromedial hypothalamic neurons control a defensive emotion state." Elife 4. doi: 10.7554/eLife.06633.
Lefler, Y., D. Campagner, and T. Branco. 2020. "The role of the periaqueductal gray in escape behavior." Curr Opin Neurobiol 60:115-121. doi: 10.1016/j.conb.2019.11.014.
Li, Z., J. X. Wei, G. W. Zhang, J. J. Huang, B. Zingg, X. Wang, H. W. Tao, and L. I. Zhang. 2021. "Corticostriatal control of defense behavior in mice induced by auditory looming cues." Nat Commun 12 (1):1040. doi: 10.1038/s41467-021-21248-7.
Lischinsky, J. E., and D. Lin. 2019. "Looming Danger: Unraveling the Circuitry for Predator Threats." Trends Neurosci 42 (12):841-842. doi: 10.1016/j.tins.2019.10.004.
Lu, B., P. Fan, M. Li, Y. Wang, W. Liang, G. Yang, F. Mo, Z. Xu, J. Shan, Y. Song, J. Liu, Y. Wu, and X. Cai. 2023. "Detection of neuronal defensive discharge information transmission and characteristics in periaqueductal gray double-subregions using PtNP/PEDOT:PSS modified microelectrode arrays." Microsyst Nanoeng 9:70. doi: 10.1038/s41378-023-00546-8.
Magierek, V., P. L. Ramos, N. G. da Silveira-Filho, R. L. Nogueira, and J. Landeira-Fernandez. 2003. "Context fear conditioning inhibits panic-like behavior elicited by electrical stimulation of dorsal periaqueductal gray." Neuroreport 14 (12):1641-4. doi: 10.1097/00001756-200308260-00020.
McNally, G. P., J. P. Johansen, and H. T. Blair. 2011. "Placing prediction into the fear circuit." Trends Neurosci 34 (6):283-92. doi: 10.1016/j.tins.2011.03.005.
Menegas, W., K. Akiti, R. Amo, N. Uchida, and M. Watabe-Uchida. 2018. "Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli." Nat Neurosci 21 (10):1421-1430. doi: 10.1038/s41593-018-0222-1.
Morgan, M. M., P. K. Whitney, and M. S. Gold. 1998. "Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray." Brain Res 804 (1):159-66. doi: 10.1016/s0006-8993(98)00669-6.
Otchy, T. M., S. B. Wolff, J. Y. Rhee, C. Pehlevan, R. Kawai, A. Kempf, S. M. Gobes, and B. P. Olveczky. 2015. "Acute off-target effects of neural circuit manipulations." Nature 528 (7582):358-63. doi: 10.1038/nature16442.
Paxinos, G., and C. Watson. 1998. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press.
Rajasethupathy, P., S. Sankaran, J. H. Marshel, C. K. Kim, E. Ferenczi, S. Y. Lee, A. Berndt, C. Ramakrishnan, A. Jaffe, M. Lo, C. Liston, and K. Deisseroth. 2015. "Projections from neocortex mediate top-down control of memory retrieval." Nature 526 (7575):653-9. doi: 10.1038/nature15389.
Ressler, R. L., and S. Maren. 2019. "Synaptic encoding of fear memories in the amygdala." Curr Opin Neurobiol 54:54-59. doi: 10.1016/j.conb.2018.08.012.
Schenberg, L. C., R. M. Povoa, A. L. Costa, A. V. Caldellas, S. Tufik, and A. S. Bittencourt. 2005. "Functional specializations within the tectum defense systems of the rat." Neurosci Biobehav Rev 29 (8):1279-98. doi: 10.1016/j.neubiorev.2005.05.006.
Silva, B. A., C. Mattucci, P. Krzywkowski, E. Murana, A. Illarionova, V. Grinevich, N. S. Canteras, D. Ragozzino, and C. T. Gross. 2013. "Independent hypothalamic circuits for social and predator fear." Nat Neurosci 16 (12):1731-3. doi: 10.1038/nn.3573.
Tsang, E., C. Orlandini, R. Sureka, A. H. Crevenna, E. Perlas, I. Prankerd, M. E. Masferrer, and C. T. Gross. 2023. "Induction of flight via midbrain projections to the cuneiform nucleus." PLoS One 18 (2):e0281464. doi: 10.1371/journal.pone.0281464.
Vianna, D. M., and M. L. Brandao. 2003. "Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear." Braz J Med Biol Res 36 (5):557-66. doi: 10.1590/s0100-879x2003000500002.
Walker, D. L., and M. Davis. 1997. "Involvement of the dorsal periaqueductal gray in the loss of fear-potentiated startle accompanying high footshock training." Behav Neurosci 111 (4):692-702. doi: 10.1037//0735-7044.111.4.692.
Wang, L., I. Z. Chen, and D. Lin. 2015. "Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors." Neuron 85 (6):1344-58. doi: 10.1016/j.neuron.2014.12.025.
Wei, P., N. Liu, Z. Zhang, X. Liu, Y. Tang, X. He, B. Wu, Z. Zhou, Y. Liu, J. Li, Y. Zhang, X. Zhou, L. Xu, L. Chen, G. Bi, X. Hu, F. Xu, and L. Wang. 2015. "Processing of visually evoked innate fear by a non-canonical thalamic pathway." Nat Commun 6:6756. doi: 10.1038/ncomms7756.
Yeh, L. F., T. Ozawa, and J. P. Johansen. 2021. "Functional organization of the midbrain periaqueductal gray for regulating aversive memory formation." Mol Brain 14 (1):136. doi: 10.1186/s13041-021-00844-0.
Yilmaz, M., and M. Meister. 2013. "Rapid innate defensive responses of mice to looming visual stimuli." Curr Biol 23 (20):2011-5. doi: 10.1016/j.cub.2013.08.015.
Zhou, Z., X. Liu, S. Chen, Z. Zhang, Y. Liu, Q. Montardy, Y. Tang, P. Wei, N. Liu, L. Li, R. Song, J. Lai, X. He, C. Chen, G. Bi, G. Feng, F. Xu, and L. Wang. 2019. "A VTA GABAergic Neural Circuit Mediates Visually Evoked Innate Defensive Responses." Neuron 103 (3):473-488 e6. doi: 10.1016/j.neuron.2019.05.027.