Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorMatthieu LouisUniversity of California, Santa Barbara, Santa Barbara, United States of America
- Senior EditorTimothy BehrensUniversity of Oxford, Oxford, United Kingdom
Reviewer #1 (Public Review):
The authors use electrophysiological and behavioral measurements to examine how animals could reliably determine odor intensity/concentration across repeated experiences. Because stimulus repetition leads to short-term adaptation evidenced by reduced overall firing rates in the antennal lobe and firing rates are otherwise concentration-dependent, there could be an ambiguity in sensory coding between reduced concentration or more recent experience. This would have a negative impact on the animal's ability to generate adaptive behavioral responses that depend on odor intensities. The authors conclude that changes in concentration alter the constituent neurons contributing to the neural population response, whereas adaptation maintains the 'activated ensemble' but with scaled firing rates. This provides a neural coding account of the ability to distinguish odor concentrations even after extended experience. Additional analyses attempt to distinguish hypothesized circuit mechanisms for adaptation but are inconclusive. A larger point that runs through the manuscript is that overall spiking activity has an inconsistent relationship with behavior and that the structure of population activity may be the more appropriate feature to consider.
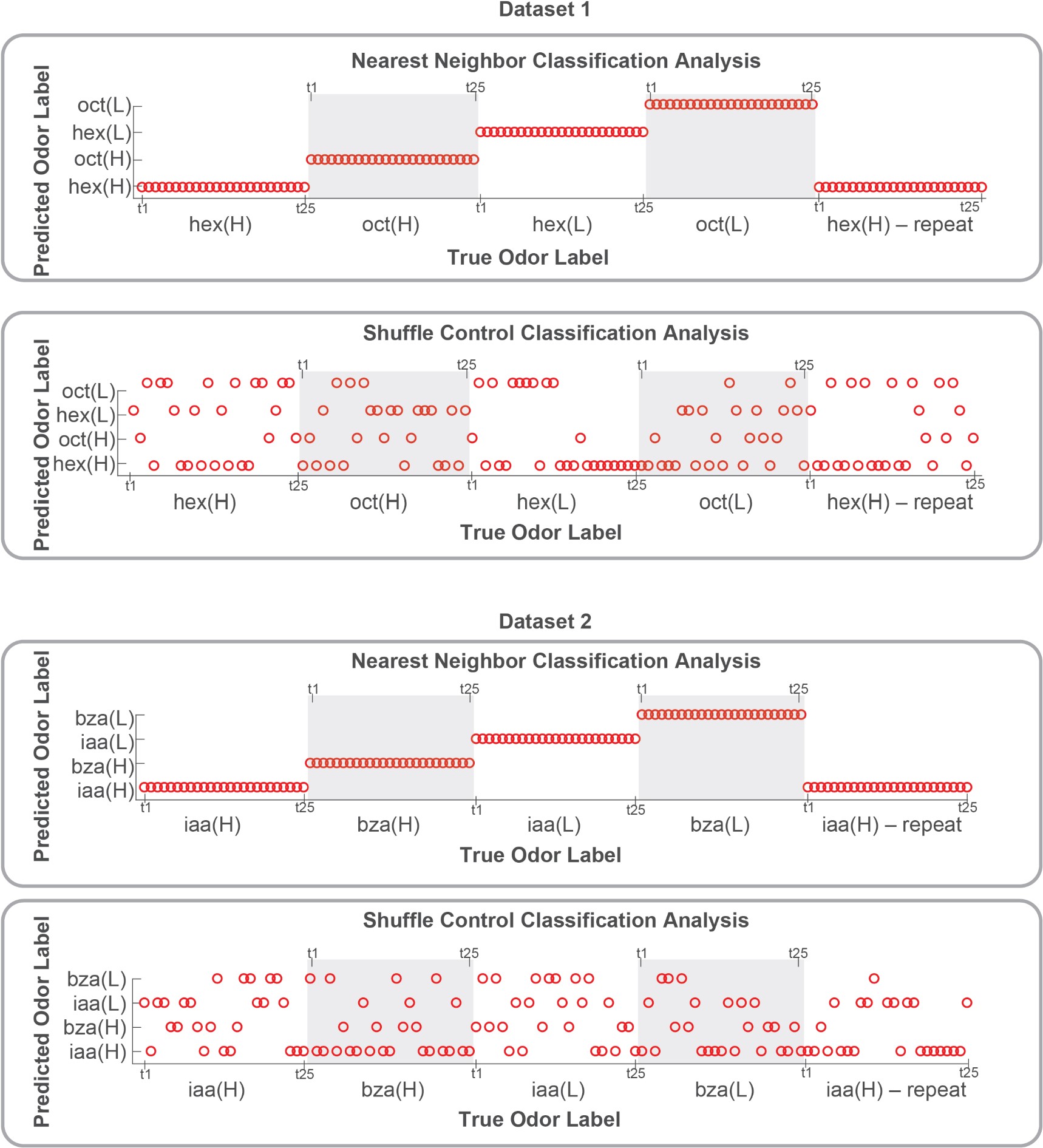
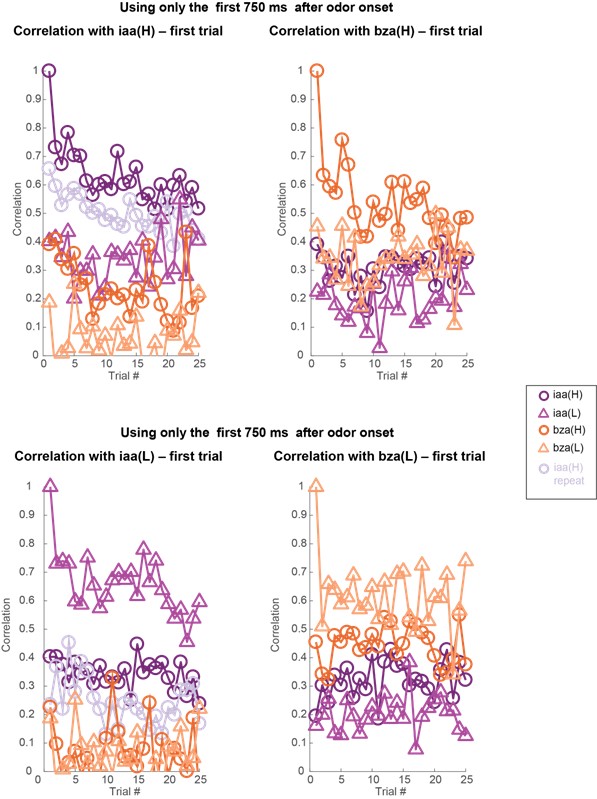
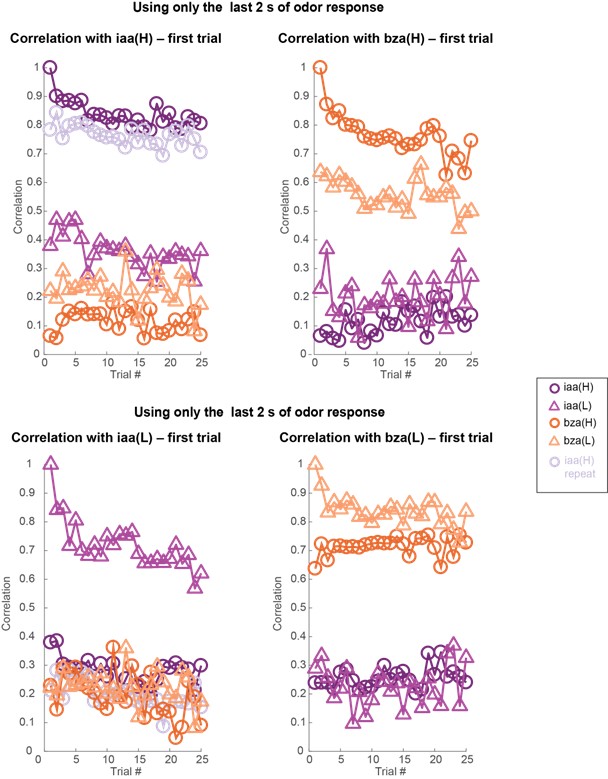
To my knowledge, the dissociation of effects of odor concentration and adaptation on olfactory system population codes was not previously demonstrated. This is a significant contribution that improves on any simple model based on overall spiking activity. The primary result is most strikingly supported by visualization of a principal components analysis in Figure 4. However, there are some weaknesses in the data and analyses that limit confidence in the overall conclusions.
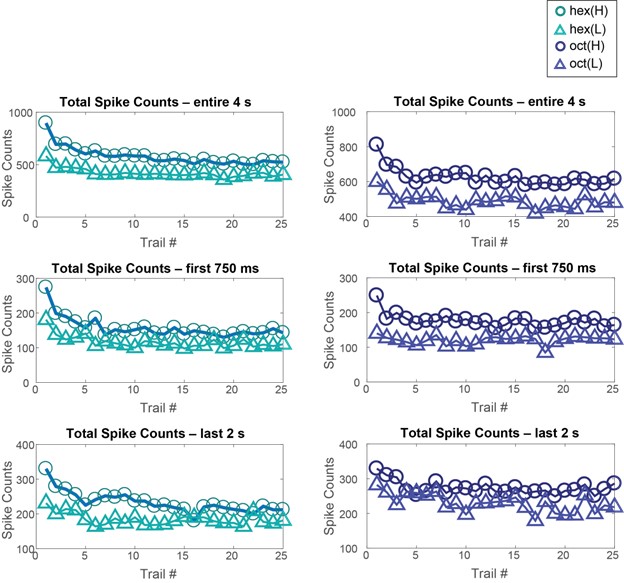
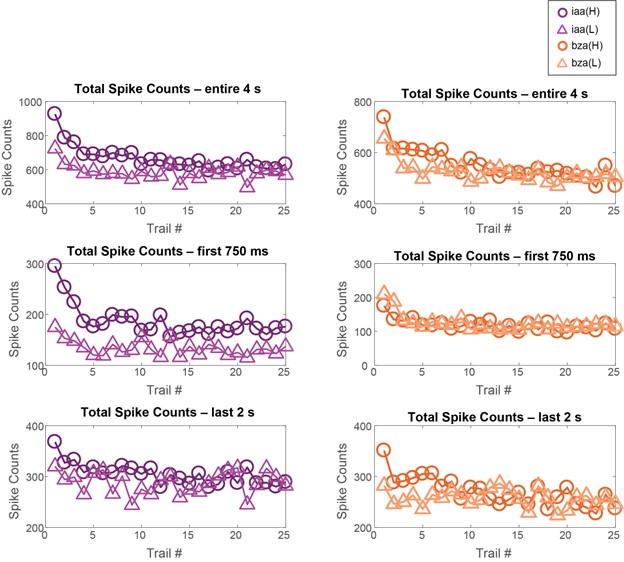
- Behavioral work interpreted to demonstrate discrimination of different odor concentrations yields inconsistent results. Only two of the four odorants follow the pattern that is emphasized in the text (Figure 1F). Though it's a priori unlikely that animals are incapable of distinguishing odor concentrations at any stage in adaptation, the evidence presented is not sufficient to reach this conclusion.
- While conclusions center on concepts related to the combination of activated neurons or the "active ensemble", this specific level of description is not directly demonstrated in any part of the results. We see individual neural responses and dimensional reduction analyses, but we are unable to assess to what extent the activated ensemble is maintained across experience.
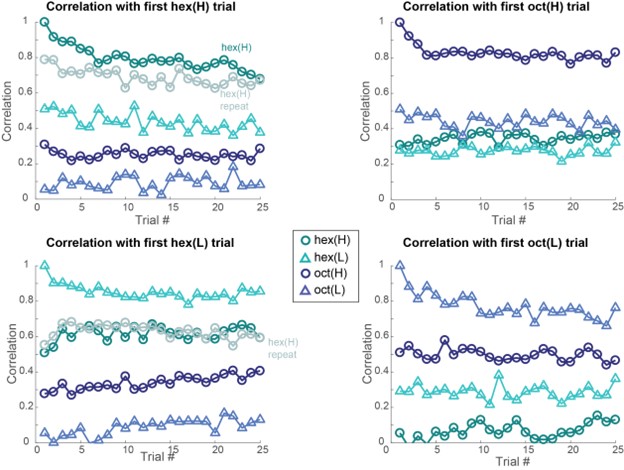
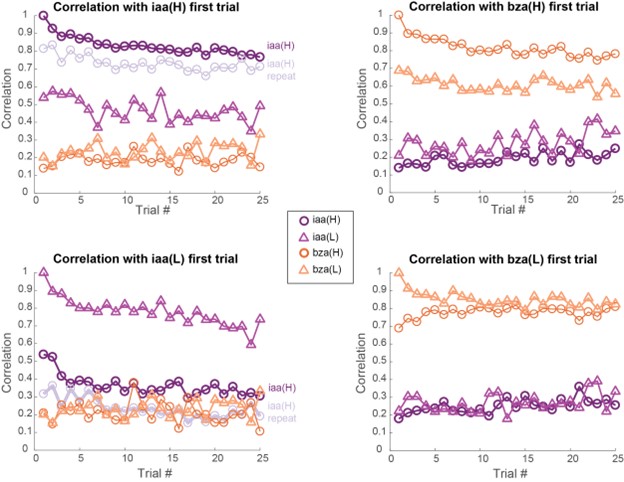
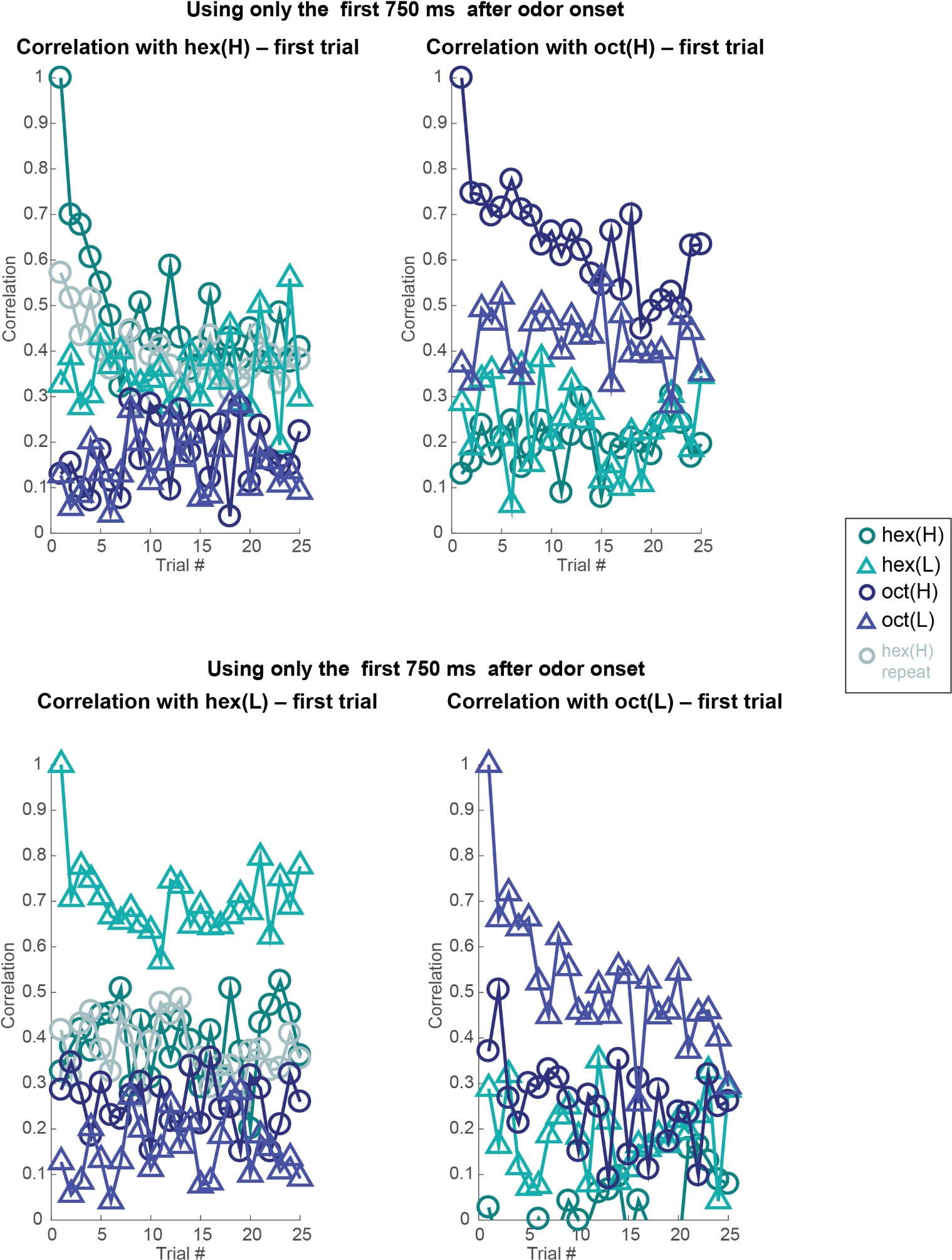
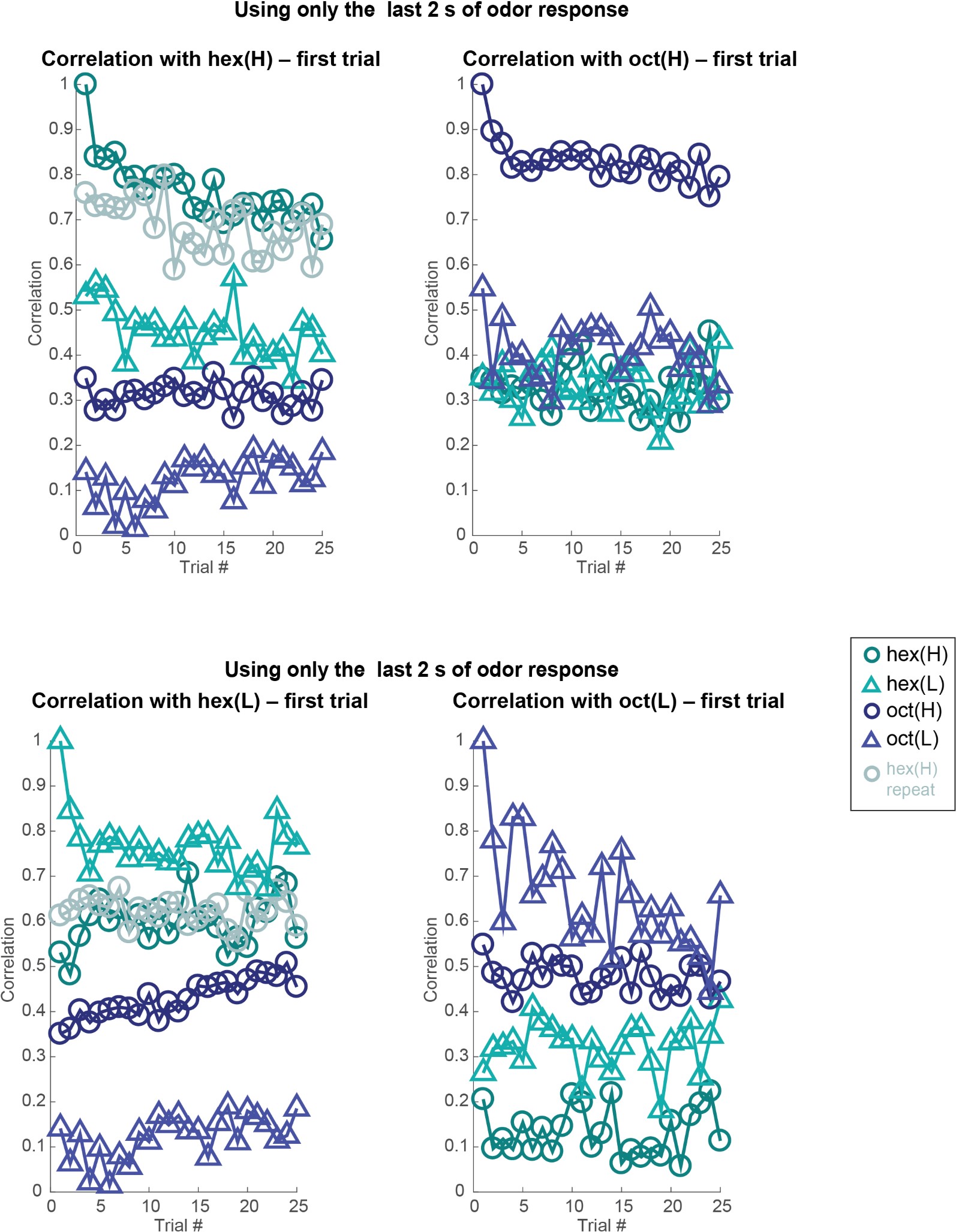
- There is little information about the variance or statistical strength of results described at the population level. While the PCA presents a compelling picture, the central point that concentration changes and adaptation alter population responses across separable dimensions is not demonstrated quantitatively. The correlation analysis that might partially address this question is presented to be visually interpreted with no additional testing.
- Results are often presented separately for each odor stimulus or for separate datasets including two odor stimuli. An effort should be made to characterize patterns of results across all odor stimuli and their statistical reliability. This concern arises throughout all data presentations.
- The relevance of the inconclusive analysis of inferred adaptation mechanisms in Figure 2d-f and the single experiment including a complex mixture in Figure 7 to the motivating questions for this study are unclear.
- Throughout the description of the results, typical standards for statistical reporting (sample size, error bars, etc.) are not followed. This prevents readers from assessing effect sizes and undermines the ability to assign a confidence to any particular conclusion.
Reviewer #2 (Public Review):
Summary:
The authors' main goal was to evaluate how both behavioral responses to odor, and their early sensory representations are modified by repeated exposure to odor, asking whether the process of adaptation is equivalent to reducing the concentration of an odor. They open with behavioral experiments that actually establish that repeated odor presentation increases the likelihood of evoking a behavioral response in their experimental subjects - locusts. They then examine neural activity patterns at the second layer of the olfactory circuit. At the population level, repeated odor exposure reduces total spike counts, but at the level of individual cells there seems to be no consistent guiding principle that describes the adaptation-related changes, and therefore no single mechanism could be identified.
Both population vector analysis and pattern correlation analysis indicate that odor intensity information is preserved through the adaptation process. They make the closely related point that responses to an odor in the adapted state are distinct from responses to lower concentration of the same odor. These analyses are appropriate, but the point could be strengthened by explicitly using some type of classification analysis to quantify the adaptation effects. e.g. a confusion matrix might show if there is a gradual shift in odor representations, or whether there are trials where representations change abruptly.
Strengths:
One strength is that the work has both behavioral read-out of odor perception and electrophysiological characterization of the sensory inputs and how both change over repeated stimulus presentations. It is particularly interesting that behavioral responses increase while neuronal responses generally decrease. Although the behavioral effect could occur fully downstream of the sensory responses the authors measure, at least those sensory responses retain the core features needed to drive behavior despite being highly adapted.
Weaknesses:
Ultimately no clear conceptual framework arises to understand how PN responses change during adaptation. Neither the mechanism (vesicle depletion versus changes in lateral inhibition) nor even a qualitative description of those changes. Perhaps this is because much of the analysis is focused on the entire population response, while perhaps different mechanisms operate on different cells making it difficult to understand things at the single PN level.
From the x-axis scale in Fig 2e,f it appeared to me that they do not observe many strong PN responses to these stimuli, everything being < 10 spikes/sec. So perhaps a clearer effect would be observed if they managed to find the stronger responding PNs than captured in this dataset.
Reviewer #3 (Public Review):
Summary:
How does the brain distinguish stimulus intensity reduction from response reductions due to adaptation? Ling et al study whether and how the locust olfactory system encodes stimulus intensity and repetition differently. They show that these stimulus manipulations have distinguishable effects on population dynamics.
Strengths:
1. Provides a potential strategy with which the brain can distinguish intensity decrease from adaptation. -- while both conditions reduce overall spike counts, intensity decrease can also changes which neurons are activated and adaptation only changes the response magnitude without changing the active ensemble.
2. By interleaving a non-repeated odor, they show that these changes are odor-specific and not a non-specific effect.
3. Describes how proboscis orientation response (POR) changes with stimulus repetition., Unlike the spike counts, POR increases in probability with stimulus. The data portray the variability across subjects in a clear way.
Weaknesses:
1. Behavior
a. While the "learning curve" of the POR is nicely described, the behavior itself receives very little description. What are the kinematics of the movement, and do these vary with repetition? Is the POR all-or-nothing or does it vary trial to trial?
b. What are the reaction times? This can constrain what time window is relevant in the neural responses. E.g., if the reaction time is 500 ms, then only the first 500 ms of the ensemble response deserves close scrutiny. Later spikes cannot contribute.
c. The behavioral methods are lacking some key information. While references are given to previous work, the reader should not be obligated to look at other papers to answer basic questions: how was the response measured? Video tracking? Hand scored?
d. Can we be sure that this is an odor response? Although airflow out of the olfactometer is ongoing throughout the experiment, opening and closing valves usually creates pressure jumps that are likely to activate mechanosensors in the antennae.
e. What is the baseline rate of PORs in the absence of stimuli?
e.What can you say about the purpose of the POR? I lack an intuition for why a fly would wiggle the maxillary palps. This is a question that is probably impossible to answer definitively, but even a speculative explanation would help the reader better understand.
2. Physiology
a. Does stimulus repetition affect "spontaneous" activity (i.e., firing in the interstimulus interval? To study this question, in Figures 2b and c, it would be valuable to display more of the pre-stimulus period, and a quantification of the stability or lability of the inter-stimulus activity.
b. When does the response change stabilize? While the authors compare repetition 1 to repetition 25, from the rasters it appears that the changes have largely stabilized after the 3rd or 4th repetition. In Figure 5, there is a clear difference between repetition 1-3 or so and the rest. Are successive repetitions more similar than more temporally-separated repetitions (e.g., is rep 13 more similar to 14 than to 17?). I was not able to judge this based on the dendrograms of Figure 5. If the responses do stabilize at it appears, it would be more informative to focus on the dynamics of the first few repetitions.
c. How do temporal dynamics change? Locust PNs have richly varied temporal dynamics, but how these may be affected is not clear. The across-population average is poorly suited to capture this feature of the activity. For example, the PNs often have an early transient response, and these appear to be timed differently across the population. These structures will be obscured in a cross population average. Looking at the rasters, it looks like the initial transient changes its timing (e.g., PN40 responses move earlier; PN33 responses move later.). Quantification of latency to first spike after stimulus may make a useful measure of the dynamics.
d. How legitimate is the link between POR and physiology? While their changes can show a nice correlation, the fact the data were taken from separate animals makes them less compelling than they would be otherwise. How feasible is it to capture POR and physiology in the same prep? This would be most helpful, but I suspect may be too technically challenging to be within scope.