Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Recommendations For The Authors):
In its current form, I would exclude the cryo-EM data from the manuscript. It does not add much and it is distracting from the excellent work that you did on the functional characterization of the variant. Alternatively, you could try to improve the resolution and see if you can get some more meaningful analysis out of the structures? I noticed that you only collected very small datasets. If you decide to pursue a higher resolution reconstruction, collecting more movies will give you a better chance to obtain a higher resolution.
We express our gratitude to the reviewer for their invaluable feedback. While acknowledging that our structure currently maintains a low resolution, it still provides valuable insights into the splice's proximity to the N412 glycan density. This proximity and low-resolution map hindered the complete modeling of all the splice residues. Notably, this structure represents the first depiction of this particular splice variant. Consequently, it lays a foundation for subsequent studies in the field, and hence, we would want to keep it in the manuscript. As per reviewers’ suggestions, we have now included comparisons of our structure with the GluK1-2a receptor structure reported recently (Mayerson et al. 2022). We do plan to carry out higher-resolution structures in the future.
I would probably also exclude the RNAseq analysis. I think that Figure 1 is fine, but the supplement 1 is not very successful in convincing me that the exon 9 is expressed mainly in early stages of brain development. In addition, the plot in Figure 1 indicates strong expression in the cerebellar cortex in 20s and 30s. If you decide to keep the data, I strongly encourage you to include more details on the analysis in the methods section.
Thanks for this insightful comment. We have now modified this section extensively for better clarity. Indeed, the expression of this variant seems to be dynamic in different brain regions. This has now been specified in the revised manuscript. Figure 1 shows the expression of GRIK1 exon 9 gene in different regions of the human brain and donor age. The supplementary figure 1 is a zoom-in on one such region, the Cerebral cortex, where we observe the maximum expression of GRIK1. In this region, we also observed higher expression of exon 9 in the early stages of development. The scales of Figure 1 (0-4 RPKM) and supplemental Figure 1(06RPKM) are different due to more expression of other exons in supplemental Figure 1 (example, we observe 4RPKM expression in the shade of red, for figure 1, whereas similar values of 4RPKM are orange-yellow in the supplemental figure1). Using Supplemental Figure 1, we wanted to show the expression of exon 9 with respect to other exons during developmental stages that prove that GluK1-1 is highly expressed in the initial stages of life. more details on the analysis in the methods section has been added now.
Additionally, there are a few minor issues in the data presentation:
(1) in Fig. 2C there seems to be a mismatch between the green dose response plot and the GluK12a trace shown. The plot reports an EC50 of 187.7 uM, whereas in the sample trace 0.25 mM agonist activates only to ~20%.
We have verified the data and statistics, confirming their consistency with the values reported in the manuscript. For Figure 2C, we present representative traces from a single cell. However, the EC50 value was calculated using Hill's equation based on averaged data from 5 cells.
(2) The axis label is misprinted in Figure 3C
Thanks. Corrected.
(3) In Fig 5 supplement 1, panel B - the 3 last labels above the western blot lanes are off so it is difficult to see which sample corresponds to which lane.
Thanks. We have corrected the figure.
Reviewer #2 (Recommendations For The Authors):
Overall I congratulate the authors of this study nicely done. It represents a large body of work.
We thank the reviewer for his/her time and positive comments.
I have several minor corrections that authors could consider for the revision of the manuscript P7. The desensitization rate of GluK1-2a was "delayed"... replace by "increased".
Corrected.
P9. Last line 0.37; P.. Add the P value.
P value has been added as suggested.
P11 authors indicate that K368/375//379/382H376-E mutant exhibit significant difference in desensitization properties in presence of NEto1, but on the 1st line of p11, they provide a P value above 0.05
We thank the reviewer for pointing out this discrepancy and have fixed the same. We have discussed two mutants that show slower desensitization when compared to GluK1-1a co-expressed with Neto1. The K to E mutant has significance, while the des value for the K368/375//379/382H376-E mutant shows the same pattern, though not significantly. We have now modified the text to explain this more clearly.
P19 the calculation of mean weighted tau TDes is not clear and should be better explained.
Thanks. We have added more details in the Methods sections. We analyzed the current decays in response to 1–2 ms or 1 s applications by employing an exponential function or the sum of two exponential functions. This analysis allowed us to derive a weighted mean τdes using the formula [(τ1 × amplitude1) + (τ2 × amplitude2)]/[amplitude1 + amplitude2]. The tau values represent the time constants obtained from the exponential fits, while the amplitudes correspond to the estimated contributions of each component to the total peak current amplitude.
[(A1 * t1) + (A2 * t2)] / (A1 + A2)
It represents the calculation of a weighted mean, where A1 and A2 are the amplitudes, and t1 and t2 are the corresponding time constants. The formula calculates the overall mean time constant by taking into account the contribution of each component to the total amplitude.
P19 the rate of recovery was obtained by fitting the one-phase association "with" exponential function. With is missing.
We have corrected this error. Thanks.
P21 which method has been used for site directed mutagenesis
Overlapping PCR was carried out for mutagenesis using the primers listed in Figure 4-table supplement 1. A ligation-free cloning approach (Zhang et al., 2017) was used. It has now been elaborated in the methodology section under Site directed mutagenesis.
P21 and 22. Provide complete reference of reagent including species of antibodies.
Thanks. We have added all the details in the methods section now.
Anti-His: Rabbit mAb #12698 (Cell Signaling Technology)
Anti-Neto1: Rabbit #SAB3500679 (Sigma Aldrich)
Anti-GFP: Mouse mAb G1546 (Sigma Aldrich)
Anti-actin: Mouse mAb A3853 (Sigma Aldrich)
P22 How much anti His antibody was used with 40microliter of protein A?
We have used 2µg/ 40uL of Protein A slurry. This has now been added to the methodology.
P23 Authors seem to have used a virus to express protein but the protocol is not given. For example what is P2 virus?
We have now modified the manuscript to include details of baculovirus generation as per the protocol described in Goehring et al. 2014. We followed the same protocol wherein the 2nd generation of virus (P2) generated in insect (SF9) cells was used for infecting suspensionadapted HEK293-T cells for large-scale GluK1-1aEM protein expression.
Reviewer #3 (Recommendations For The Authors):
Major concerns:
(1) The effect of the splice insert on Gluk1 regulation by Neto proteins is not fully clear. For example, experiments in Fig. 3G indicate that the desensitization time for Gluk1-1a + Neto2 is ~32ms. This value is half compared with data obtained from whole-cell experiments shown in Fig. 3A (~70ms). What is the reason for this discrepancy? If variability is observed between experiments, I wonder how valid are the comparisons made in panel A between GluK11a+Neto2 vs GluK1-2a+Neto2 groups. In the case of recovery analysis, authors found significant differences comparing both groups in the presence of Neto (Fig. 3B) but recovery times are not identic for Gluk1-1a vs Gluk1-2a (without Neto). Thus, I wonder if the fold change related to the control group (without Neto) is different.
We appreciate your detailed feedback, which has allowed us to clarify and reinforce the validity of our experimental findings. Different recording configurations (e.g., outside-out patch (Fig. 3G) versus whole-cell recordings (Fig. 3A) have been used. Whole-cell recordings average responses over a larger membrane area and also have slower solution exchange times compared to outside-out patch recordings. This may have contributed to the variability in desensitization times. However, similar trends in our whole cell vs. outside-out patch recordings were observed. Further, all the data except those presented in Figs 3G and 3H are from whole-cell recordings. We have performed multiple independent experiments and utilized rigorous statistical analyses to validate our comparisons. We report mean values with standard deviations or confidence intervals to provide a more accurate representation of the data.
Neto1 significantly speeds up the recovery from desensitization for both variants, with a more pronounced effect on GluK1-1a (GluK1-1a +Neto1: 0.68 s) compared to GluK1-2a (GluK1-2a +Neto1: 1.15 s). The recovery times are not identical for the two variants, likely due to the presence of splice insert in GluK1-1a. Neto2, on the other hand, slows recovery for both variants without significant differential effects. However, the recovery rate from the desensitized state is faster for GluK1-1 compared to GluK1-2a alone, although insignificant (without Neto).
In the case of the glutamate concentration-response curve (Fig. 3C), EC50 values for Neto1 and Neto2 are relatively the same, but this approach on its own does not provide insights about the role of the splice insert. Previous experiments with the Gluk1 reveal differences between EC50 in the presence of Neto1 or 2 (Fisher, 2015), suggesting that the insert could regulate glutamate binding affinity, but still, this point is not directly demonstrated in this work.
Thanks for this insightful comment. Indeed, we cannot conclude that splice residues directly affect glutamate sensitivity and have modified the text accordingly. The Fisher paper demonstrated that both Neto1 and Neto2 can influence glutamate sensitivity in GluK1-2a, with EC50 values of 124.6 ± 16.2 µM. Specifically, in the presence of Neto1 and Neto2, the EC50 values are 4.4 ± 0.4 µM and 13.7 ± 4.2 µM, respectively, indicating a noticeable effect though not substantially different for GluK1-2a coexpressed with either Neto1 and Neto2. Our observation for the GluK1-1a has been similar, with both Neto1 and Neto2 showing a leftward shift.
(2) Similar to the previous point, a proper interpretation of mutant data is missing in the manuscript. From current data, it is difficult to visualize the role of the insert on Netodependent regulation, mainly, because of the fact that some mutations alone affect Gluk1-1 channel properties. The authors conclude their data by stating that "while the modulation of the receptor by Neto 1 is affected by mutations in splice insert, the modulation by Neto 2 remains largely unaffected" (Page 13). However, this statement is confusing since the co-expression of Gluk1-1a with Neto2 (Fig. 5) prevents the effect caused by mutation K368 alone (Fig. 4), indicating that modulations by Neto 2 are indeed potentially affected by the mutations. Please, clarify. Also, the effect of the K368/375/379/382H376-E mutant on Neto modulation (pink bar in Fig. 5) is impossible to interpret properly since the effect of the mutation alone is not shown in the manuscript.
Thanks for seeking this important clarification. It is indeed true that splice residue mutations themselves affect the receptor functional properties in comparison to the wild-type receptors. For the sake of clarity, we have presented the effect of splice mutants on receptor properties separately from the effect of mutations on modulation by Neto proteins. Figure 4 demonstrates a comparison between wild-type and mutant receptors without the Neto proteins, showcasing different kinetic properties, while Figure 5 provides detailed information on the role of the insert in Neto-dependent regulation.
It’s true we could not record the effect of the K368/375/379/382H376-E mutant alone or when coexpressed with Neto 2 due to low peak amplitudes (mentioned in Table 1) that prevented reliable comparisons. However, robust currents were observed when the same mutant was coexpressed with Neto1, and hence comparisons were shown for this mutant with GluK1-1a wild-type + Neto1.
We have now modified the statement "while the modulation of the receptor by Neto 1 is affected by mutations in splice insert, the modulation by Neto 2 remains largely unaffected" and the last paragraph as follows:
“Neto1 appears to have more pronounced effects on the mutant receptors compared to Neto2. Specifically, Neto1 significantly slowed desensitization for the K368-E mutant, accelerated recovery from desensitization for K368-E and K368/375/379/382H376-E mutants, increased agonist efficacy for K368-E and K375/379/382H376-E mutants, and altered rectification properties for K368E and K368/375/379/382H376-E mutants. In contrast, Neto2 had fewer significant effects on the mutant receptors, with the main impact being an increase in agonist efficacy for the K368-E mutant. Notably, Neto2 did not significantly affect desensitization, recovery from desensitization, or rectification properties of the mutant receptors when compared with wildtype GluK1-1a coexpressed with Neto2. These findings suggest that the splice residues in GluK1-1a differentially influence receptor modulation by Neto1 and Neto2, with Neto1 showing more extensive modulation of the mutant receptors' functional properties.”
(3) An open question after reading this interesting work is if the proposed change in Neto regulation because of the splice insert is due to changes in Gluk1-Neto interactions or because the rearrangement after interaction with Neto proteins is different. Pull-down experiments (Fig 5 Sup.1) suggest that the splice insert and all the mutants tested do not prevent interaction with Neto proteins. I wonder if the authors could complement their data with a quantitative approach/analysis to demonstrate if the splice insert and the mutants affect Neto1/2 interactions (as expected for the rationale when creating the mutants).
Thank you for this insightful suggestion. You raise an important point about distinguishing between changes in GluK1-Neto interactions and potential differences in receptor rearrangement after Neto binding. While our pull-down experiments suggest that the splice insert and mutants don't prevent Neto interactions (probably due to a larger interaction interface all along the receptor), a quantitative approach would indeed provide more nuanced information. In future studies, we do plan to perform a quantitative approach like Surface plasmon resonance to assess the changes in interactions upon mutations in the splice and/or Neto proteins in different states of the receptor. In addition, obtaining cryo-EM structures of GluK1 splice variants in complex with Neto1 and Neto2 would provide crucial insights into their interaction interfaces and any conformational changes induced by binding.
(4) Related to the Gluk1-1a structure, the authors state that the overall structure is similar to the one without the insert (page 14); however, this is not properly shown in the manuscript. Even if the overall architecture of the channel is the same, authors should make a proper/adequate comparison between both structures/domains to support their claims. Also, one should expect that the insertion of 15 amino acids would affect in some way the closing neighboring domains. The differential effect of the splice insert on glutamate and kainate EC50 values (Fig. 2 and Fig. 2 sup.1), suggests that the insert could introduce a sort of rearrangement in the binding domain. Thus, I wonder if a more elaborated analysis of the current structural data could reveal some structural insights that would explain the specific functional differences due to the splice insert. If the low resolution and the missing residues avoid making some comparisons and establish differences between sidechain orientations, still, a proper comparison between the domain backbones would be helpful to validate the author's statement at least. Also, I wonder if the changes could be resolved better in a closed state or APO structure, instead of the desensitized structure. Finally, are the structures obtained in DDM and nanodiscs similar?
As per the reviewer’s suggestion, we have now added a new figure in the supplementary information, “Figure 6-figure supplement 9,” where we show a superimposition of GluK11aEM (detergent-solubilized or reconstituted in nanodiscs) and GluK1-2a (PDB:7LVT; silver) showing overall conservation of the structures in the desensitized state.
As evident from the figure and rmsd values mentioned above, we do not observe significant movements at both ATD and LBD layers of GluK1-1a with respect to GluK1-2a. Also as can be observed the DDM solubilized and nanodisc reconstituted GluK1-1a (Panel A) are very similar with a rmsd of ~2.19Å across all the 2664 Calpha atom pairs. Due to low resolution of our structures, we have refrained from carrying out detailed structural comparisions.
Our efforts to capture the closed state or apo state structures have failed due to either severe orientation bias (only top views) or increased heterogeneity.
(5) Methods section lacks relevant information for proper data interpretation as well as for replicating some experiments in the future. For example:
A) The experimental design to determine the rectification index with a Ramp protocol is not clear: 1) Why the authors applied a ramp protocol if receptors desensitize along the time? Please clarify the protocol.
Ramp protocols were used only for the wild-type receptors to compare their voltage-dependent behavior, as this was the first study to compare the two splice variants. All kainate receptors (GluK1-GluK5) desensitize over time. However, their rectification properties have been studied previously (both the absence and presence of Neto proteins) using Ramp protocols as they are faster than step protocols.
B) Are polyamines included in the solutions to perform the rectification assays?
No, polyamines were not added to the intracellular solution, and the effect of the endogenous polyamine block was measured. This has now been specified in the results as well as the methods section.
C) It is not clear if the experiments to calculate IK/IG ratios were performed in the same preparation (This is, the same cell was stimulated with glutamate and then kainate or vice versa).
Indeed, the current responses for glutamate vs kainate are performed in the same cell (the same cell was stimulated by glutamate then kainate) so that the responses can be compared. It’s now been specified in the methods section.
D) The experimental design for calculating recovery is not clear.
We employed a double pulse protocol to measure receptor recovery. The protocol involved applying two consecutive pulses of agonist stimulation to the receptor. Initially, we applied a brief agonist pulse to activate the receptor, followed by a specific recovery period. After the recovery period, we administered a second agonist pulse to assess the receptor's recovery response. The receptor's recovery was determined by comparing the response amplitude of the second pulse to that of the first pulse, providing valuable insights into the receptor's recovery kinetics. Recovery rates were calculated with single exponential association fits in Prism. We have now modified the text for better clarity.
E) Please indicate the species used for both functional and Cryo-EM (rat Gluk1 isoform?).
Thanks for pointing this out. We have now specified in relevant methodology sections that Rattus norvegicus GluK1 and Neto proteins were used in this study.
F) Please describe the nanodisc reconstitution protocol and how the nanodisc protein was purified, if appropriate.
The MSP1E3D1 was purified by following the protocol given by the Sligar group in 2014 (doi: 10.1016/S0076-6879(09)64011-8). The nanodisc reconstitution protocol has now been elaborated in the revised manuscript.
G) Site-directed mutagenesis methodology is incomplete. Please check.
We have now elaborated this section to include more details.
Minor concerns:
(1) Authors state that splice residues are ~30A away from the TM domain. Currently, there is no friendly representation showing the localization of the splice in the structure, besides Fig.6E. The manuscript could benefit itself if authors include a better 3D representation or a scheme to highlight the position of the splice relative to critical domains.
Thanks for pointing this out. The distance between TRP 381 CA (ATD) and LEU 636 CA (TM3) is 92.10 Å. We have changed the value in the text to ~92 Å.
Author response image 1.
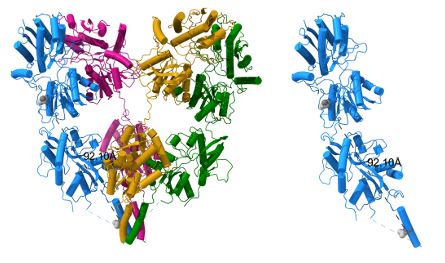
(2) Authors mention that mutations in the insert to alanine show normal traffic to the plasma membrane but low current amplitude. Then, I wonder if single-channel conductance, mean open time or open probability is affected by the splice insert. Showing the effects of the insert on single-channel properties would strengthen the manuscript's quality.
It is a good suggestion. However, as can be observed from our whole cell or outside out patch data, we obtained low peak amplitudes (<50 pA) for many of our receptor-only constructs and also suffered from high SEM for some recordings due to heterogeneity between cells of the same population. The suggestion to study the single channel properties of these receptors is considered for future experiments
(3) It is unclear how the insert or the mutations specifically affect glutamate- or kainate-induced responses because authors analyze IK/IG ratios only. Maybe authors could consider including an analysis of the role of the insert on specific glutamate- or kainate-induced response to gain insights about ligand selectivity.
All the values have been included in the excel for raw data. We have included the desensitization kinetics of mutant receptors in the presence of glutamate and compared it to the wild type GluK1-1a. Kainate induced responses were very heterogenous (high SEM for % desensitization) and hence have not been included in the main data.
(4) Please be consistent with nomenclature along the manuscript to avoid confusion. For example, Are Gluk-1-1 and Gluk-1-1a referring to the same variant?
GluK1-1 has been used in the abstract and the introduction where we introduce the N-terminal splice variant which either has the 15 residues (termed as GluK1-1) or lacks it (GluK1-2). The C- terminal splice variants for GluK1 are named as “a-d”, with “a” being the smallest Cterminal domain variant. Later in the manuscript, we have used only GluK1-1a terminology to represent the ATD splice variant with shortest C-terminal domain.
The introduction and spatiotemporal results talk about the GluK1-1 receptors wherein the
(5) Legend figure 2: Repeated phrase should be removed. Please check.
(6) Page 8: "This is similar to the effect observed in GluK1-2 receptors whereby the glutamate EC50 was shown to increase by Neto proteins [Neto1: 34-fold and Neto2: 7.5-fold (Palacios-Filardo et al., 2016) and Neto1/2: 10-30X (Fisher, 2015)]". It seems that values from Fisher's paper are backward. Please correct.
(7) Page 9. Second paragraph. Spelling mistake when referring to Fig. 3G.
Thanks for pointing out the inadvertent errors; we have now corrected all of them.
(8) Figure 3: The title in Y axis overlaps with the figure. Please check.
We have corrected the error.
(9) Page 10: "In addition, K375/379/382H376-E mutant also exhibited a slowdown in the recovery (K375/379/382H376-E: 4.83 {plus minus} 0.31 s P=0.2774) (Figure 4C; Table 1)." Statistical analysis indicates this is not correct. Please tone down this statement. For example: "...mutant also exhibited a trend to a slowdown in the recovery although differences do not reach statistical significance".
Thanks. We have modified the statement as suggested.
(10) Page 11: "and a reduction was observed for K375/379/382H376-E receptors (1.17 {plus minus} 0.28 P=0.3733) compared to wild-type (Figure 4D; Table 1)." Same issue as the previous minor comment.
Thanks. We have modified the statement as suggested.
(11) Page 11: "We observed that mutants K368-E and K368/375/379/382H376-E, desensitize significantly slower in the presence of Neto1" This statement is not true for K368/375/379/382H376-E mutant. Please correct.
Thanks. We have modified the statement as suggested and specified the difference.
(12) Legend Figure 4. Colored asterisks are not clear in the figure. Please check.
Thanks. The reference to colored asterisks has been removed from the legend as they are not used.
(13) Representative data shown in Fig 5 sup.2A do not match very well with the final quantification shown in Fig 5A. Please check. Also, the authors state in the result section (page 10) that data shown in Fig. 5A indicate that "GluK1-1a modulation by Neto 1 is influenced by the splice residues". This could be true only for residue K368; however, this is not so obvious since the two mutants containing K368E are inconsistent. Please check and clarify.
Only representative traces are shown in Fig 5 sup 2 A. However, the quantification shown in Fig 5 A is from multiple cells. We have rechecked all the data and found it to be consistent. We have rewritten this section and modified it for better clarity.
(14) Figure 6-supplement 2: Please incorporate missing values of MW standards in panel B.
Thanks. We have modified the figure to include values for MW standards.
(15) It is not clear the rationale for showing construct C552Y C557V C575S in Fig. 6 sup.3, panel A. This mutant is not mentioned in the manuscript.
It has been mentioned in the methodology section under “Construct design for expression and purification of rat GluK1-1aEM”. It (C552Y C557V C576S) is one of the constructs used in optimizations that were checked for good protein yields. Based on FSEC protein profiles, we used C552Y, C557V (2X Cys mutant) as GluK1-1aEM, which is mentioned in the same section.
(16) Fig. 6 sup.4 Not clear what does mean w.r.c. Please specify in the legend.
With respect to (w. r. t.) has been specified in the manuscript.
(17) Suggestion to improve data presentation in Fig. 4D and Fig. 3 sup.1B: For easier comparison of IK/IG ratios, representative traces for kainate and glutamate in the same group could be shown using the same Y-scale.
It has been purposely shown with two different Y-scales due to the differences in peak amplitudes in the presence of glutamate or kainate.
(18) Fig. 3 sup.1A: Based on the figure legend, horizontal bars representing the application of glutamate are not consistent with time scale bars. Please, check. In the same figure, panel B, the representative traces shown for GluK-1a-Neto1 are not consistent with IK/IG ratio shown in Fig. 3D.
Thanks, we have corrected the horizontal bars representing glutamate application. The representative traces shown for GluK-1a-Neto1 were rechecked and are consistent with the IK/IG ratio shown in Fig. 3D.
(19) I wonder if the authors could discuss the lack of Neto1 effect on the wild type Gluk1-2a channel, as proposed previously.
Sheng et al., 2015 showed that Neto1 enhances the desensitization onset of GluK1. However, it is unclear which GluK1 splice variants were used in that study. GluK1 has several splice variants, but in the present study, we specifically compared GluK1-1a and 2a. In our case, we did not observe the effect of Neto1 on wild-type GluK1-2a in either of the two techniques (whole cell and outside-out patch) we utilized for our study. However, as can be observed from our data, the GluK1-2a receptor alone shows a faster desensitization kinetics than the previous study (Copits et al., 2011). The differences could stem from different experimental conditions such as constructs, recording conditions used etc.
Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. Journal of Neuroscience. 2011 May 18;31(20):7334-40.
Sheng N, Shi YS, Lomash RM, Roche KW, Nicoll RA. Neto auxiliary proteins control both the trafficking and biophysical properties of the kainate receptor GluK1. Elife. 2015 Dec 31;4:e11682. doi: 10.7554/eLife.11682. PMID: 26720915; PMCID: PMC4749551.




