Peer review process
Not revised: This Reviewed Preprint includes the authors’ original preprint (without revision), an eLife assessment, public reviews, and a provisional response from the authors.
Read more about eLife’s peer review process.Editors
- Reviewing EditorBrice BathellierCentre National pour la Recherche Scientifique et Technique (CNRST), Paris, France
- Senior EditorBarbara Shinn-CunninghamCarnegie Mellon University, Pittsburgh, United States of America
Reviewer #1 (Public Review):
The inferior colliculus (IC) is the central auditory system's major hub. It integrates ascending brainstem signals to provide acoustic information to the auditory thalamus. The superficial layers of the IC ("shell" IC regions as defined in the current manuscript) also receive a massive descending projection from the auditory cortex. This auditory cortico-collicular pathway has long fascinated the hearing field, as it may provide a route to funnel "high-level" cortical signals and impart behavioral salience upon an otherwise behaviorally agnostic midbrain circuit.
Accordingly, IC neurons can respond differently to the same sound depending on whether animals engage in a behavioral task (Ryan and Miller 1977; Ryan et al., 1984; Slee & David, 2015; Saderi et al., 2021; De Franceschi & Barkat, 2021). Many studies also report a rich variety of non-auditory responses in the IC, far beyond the simple acoustic responses one expects to find in a "low-level" region (Sakurai, 1990; Metzger et al., 2006; Porter et al., 2007). A tacit assumption is that the behaviorally relevant activity of IC neurons is inherited from the auditory cortico-collicular pathway. However, this assumption has never been tested, owing to two main limitations of past studies:
Prior studies could not confirm if data were obtained from IC neurons that receive monosynaptic input from the auditory cortex.
Many studies have tested how auditory cortical inactivation impacts IC neuron activity; the consequence of cortical silencing is sometimes quite modest. However, all prior inactivation studies were conducted in anesthetized or passively listening animals. These conditions may not fully engage the auditory cortico-collicular pathway. Moreover, the extent of cortical inactivation in prior studies was sometimes ambiguous, which complicates interpreting modest or negative results.
Here, the authors' goal is to directly test if auditory cortex is necessary for behaviorally relevant activity in IC neurons. They conclude that surprisingly, task relevant activity in cortico-recipient IC neuron persists in absence of auditory cortico-collicular transmission. To this end, a major strength of the paper is that the authors combine a sound-detection behavior with clever approaches that unambiguously overcome the limitations of past studies.
First, the authors inject a transsynaptic virus into the auditory cortex, thereby expressing a genetically encoded calcium indicator in the auditory cortex's postsynaptic targets in the IC. This powerful approach enables 2-photon Ca2+ imaging from IC neurons that unambiguously receive monosynaptic input from auditory cortex. Thus, any effect of cortical silencing should be maximally observable in this neuronal population. Second, they abrogate auditory cortico-collicular transmission using lesions of auditory cortex. This "sledgehammer" approach is arguably the most direct test of whether cortico-recipient IC neurons will continue to encode task-relevant information in absence of descending feedback. Indeed, their method circumvents the known limitations of more modern optogenetic or chemogenetic silencing, e.g. variable efficacy.
I also see three weaknesses which limit what we can learn from the authors' hard work, at least in the current form. I want to emphasize that these issues do not reflect any fatal flaw of the approach. Rather, I believe that their datasets likely contain the treasure-trove of knowledge required to completely support their claims.
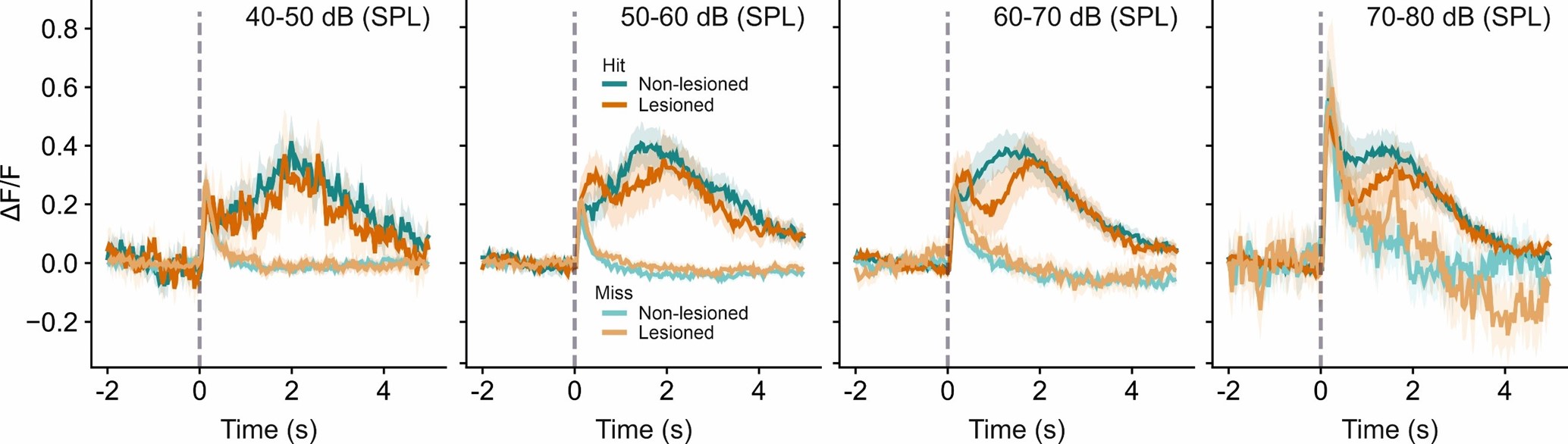
1. The conclusion of this paper requires the following assumption to be true: That the difference in neural activity between Hit and Miss trials reflects "information beyond the physical attributes of sound." The data presentation complicates asserting this assumption. Specifically, they average fluorescence transients of all Hit and all Miss trials in their detection task. Yet, Figure 3B shows that mice's d' depends on sound level, and since this is a detection task the smaller d' at low SPLs presumably reflects lower Hit rates (and thus higher Miss rates). As currently written, it is not clear if fluorescence traces for Hits arise from trials where the sound cue was played at a higher sound level than on Miss trials. Thus, the difference in neural activity on Hit and Miss trials could indeed reflect mice's behavior (licking or not licking). But in principle could also be explained by higher sound-evoked spike rates on Hit compared to Miss trials, simply due to louder click sounds. Indeed, the amplitude and decay tau of their indicator GCaMP6f is non-linearly dependent on the number and rate of spikes (Chen et al., 2013), so this isn't an unreasonable concern.
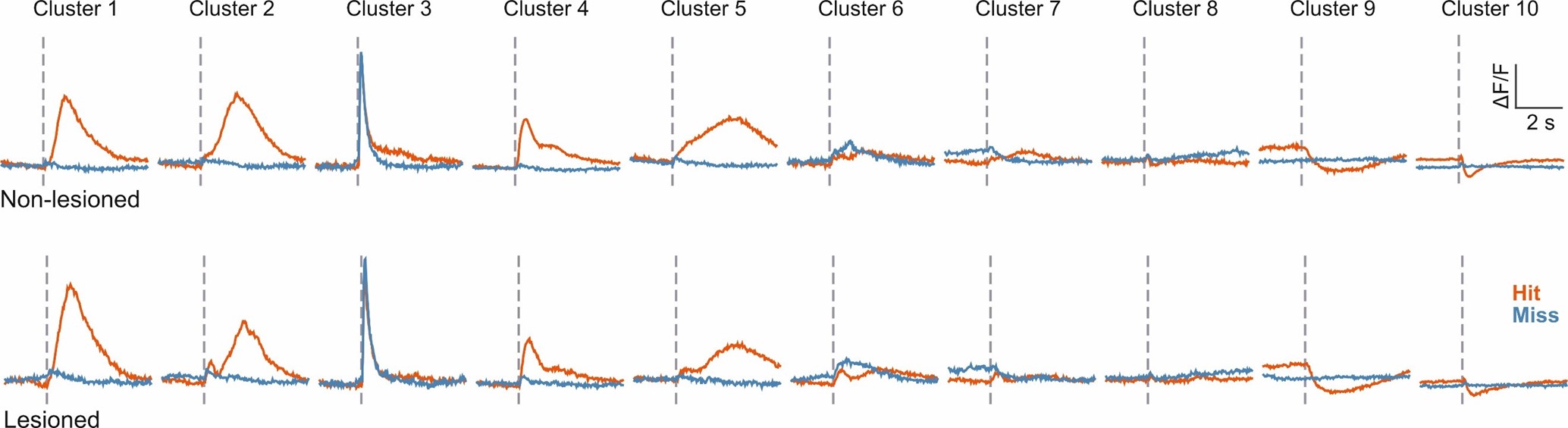
2. The authors' central claim effectively rests upon two analyses in Figures 5 and 6. The spectral clustering algorithm of Figure 5 identifies 10 separate activity patterns in IC neurons of control and lesioned mice; most of these clusters show distinct activity on averaged Hit and Miss trials. They conclude that although the proportions of neurons from control and lesioned mice in certain clusters deviates from an expected 50/50 split, neurons from lesioned mice are still represented in all clusters. A significant issue here is that in addition to averaging all Hits and Miss trials together, the data from control and lesioned mice are lumped for the clustering. There is no direct comparison of neural activity between the two groups, so the reader must rely on interpreting a row of pie charts to assess the conclusion. It's unclear how similar task relevant activity is between control and lesioned mice; we don't even have a ballpark estimate of how auditory cortex does or does not contribute to task relevant activity. Although ideally the authors would have approached this by repeatedly imaging the same IC neurons before and after lesioning auditory cortex, this within-subjects design may be unfeasible if lesions interfere with task retention. Nevertheless, they have recordings from hundreds to thousands of neurons across two groups, so even a small effect should be observable in a between-groups comparison.
3. In Figure 6, the authors show that logistic regression models predict whether the trial is a Hit or Miss from their fluorescence data. Classification accuracy peaks rapidly following sound presentation, implying substantial information regarding mice's actions. The authors further show that classification accuracy is reduced, but still above chance in mice with auditory cortical lesions. The authors conclude from this analysis task relevant activity persists in absence of auditory cortex. In principle I do not disagree with their conclusion.
The weakness here is in the details. First, the reduction in classification accuracy of lesioned mice suggests that auditory cortex does nevertheless transmit some task relevant information, however minor it may be. I feel that as written, their narrative does not adequately highlight this finding. Rather one could argue that their results suggest redundant sources of task-relevant activity converging in the IC. Secondly, the authors conclude that decoding accuracy is impaired more in partially compared to fully lesioned mice. They admit that this conclusion is at face value counterintuitive, and provide compelling mechanistic arguments in the Discussion. However, aside from shaded 95% CIs, we have no estimate of variance in decoding accuracy across sessions or subjects for either control or lesioned mice. Thus we don't know if the small sample sizes of partial (n = 3) and full lesion (n = 4) groups adequately sample from the underlying population. Their result of Figure 6B may reflect spurious sampling from tail ends of the distributions, rather than a true non-monotonic effect of lesion size on task relevant activity in IC.
Reviewer #2 (Public Review):
Summary:
This study takes a new approach to studying the role of corticofugal projections from auditory cortex to inferior colliculus. The authors performed two-photon imaging of cortico-recipient IC neurons during a click detection task in mice with and without lesions of auditory cortex. In both groups of animals, they observed similar task performance and relatively small differences in the encoding of task-response variables in the IC population. They conclude that non-cortical inputs to the IC provide can substantial task-related modulation, at least when AC is absent.
Strengths:
This study provides valuable new insight into big and challenging questions around top-down modulation of activity in the IC. The approach here is novel and appears to have been executed thoughtfully. Thus, it should be of interest to the community.
Weaknesses:
There are, however, substantial concerns about the interpretation of the findings and limitations to the current analysis. In particular, Analysis of single unit activity is absent, making interpretation of population clusters and decoding less interpretable. These concerns should be addressed to make sure that the results can be interpreted clearly in an active field that already contains a number of confusing and possibly contradictory findings.
Reviewer #3 (Public Review):
Summary:
This study aims to demonstrate that cortical feedback is not necessary to signal behavioral outcome to shell neurons of the inferior colliculus during a sound detection task. The demonstration is achieved by the observation of the activity of cortico-recipient neurons in animals which have received lesions of the auditory cortex. The experiment shows that neither behavior performance nor neuronal responses are significantly impacted by cortical lesions except for the case of partial lesions which seem to have a disruptive effect on behavioral outcome signaling.
Strengths:
The experimental procedure is based on state of the art methods. There is an in depth discussion of the different effects of auditory cortical lesions on sound detection behavior.
Weaknesses:
The analysis is not documented enough to be correctly evaluated. Have the authors pooled together trials with different sound levels for the key hit vs miss decoding/clustering analysis? If so, the conclusions are not well supported, as there are more misses for low sound levels, which would completely bias the outcome of the analysis. It would possible that the classification of hit versus misses actually only reflects a decoding of sound level based on sensory responses in the colliculus, and it would not be surprising then that in the presence or absence of cortical feedback, some neurons responds more to higher sound levels (hits) and less to lower sound levels (misses). It is important that the authors clarify and in any case perform an analysis in which the classification of hits vs misses is done only for the same sound levels. The description of feedback signals could be more detailed although it is difficult to achieve good temporal resolution with the calcium imaging technique necessary for targeting cortico-recipient neurons.