Author Response:
The following is the authors’ response to the original reviews.
eLife assessment
This study presents potentially useful findings describing how activity in the corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus modulates sevoflurane anesthesia, as well as a phenomenon the authors term a "general anesthetic stress response". The technical approaches are solid and the data presented are largely clear. However, the primary conclusion, that the PVHCRH neurons are a mechanism of sevoflurane anesthesia, is inadequately supported.
We appreciate the editors and reviewers for their thorough assessment and constructive feedback. We have provided clarifications and updated the manuscripts to better interpret our results, please see below. As for the primary conclusion, we revised it as PVH CRH neurons potently modulate states of anaesthesia in sevoflurane general anesthesia, being a part of anaesthesia regulatory network of sevoflurane.
Combined Public Review:
This study describes a group of CRH-releasing neurons, located in the paraventricular nucleus of the hypothalamus, which, in mice, affects both the state of sevoflurane anesthesia and a grooming behavior observed after it. PVH-CRH neurons showed elevated calcium activity during the post-anesthesia period. Optogenetic activation of these PVH-CRH neurons during sevoflurane anesthesia shifts the EEG from burst-suppression to a seemingly activated state (an apparent arousal effect), although without a behavioral correlate. Chemogenetic activation of the PVH-CRH neurons delays sevoflurane-induced loss of righting reflex (another apparent arousal effect). On the other hand, chemogenetic inhibition of PVH-CRH neurons delays recovery of the righting reflex and decreases sevoflurane-induced stress (an apparent decrease in the arousal effect). The authors conclude that PVH-CRH neurons are a common substrate for sevoflurane-induced anesthesia and stress. The PVH-CRH neurons are related to behavioral stress responses, and the authors claim that these findings provide direct evidence for a relationship between sevoflurane anesthesia and sevoflurane-mediated stress that might exist even when there is no surgical trauma, such as an incision. In its current form, the article does not achieve its intended goal.
Thank you for the detailed review. We have carefully considered your comments and have revised the manuscript to provide a clearer interpretation of our findings. Our findings indicate that PVH CRH neurons integrate the anesthetic effect and post-anesthesia stress response of sevoflurane (GA), providing new evidence for understanding the neuronal regulation of sevoflurane GA and identifying a potential brain target for further investigation into modulating the post-anesthesia stress response. However, we did not propose that there was a direct relationship between sevoflurane anesthesia and sevoflurane-mediated stress in the absence of incision. Our results mainly concluded that PVH CRH neurons integrate the anaesthetic effect and post-anaesthesia stress response of sevoflurane GA, which offers new evidence for the neuronal regulation of sevoflurane GA and provides an important but ignored potential cause of the post-anesthesia stress response.
Strengths:
The manuscript uses targeted manipulation of the PVH-CRH neurons, and is technically sound. Also, the number of experiments is substantial.
Thank you.
Weaknesses:
The most significant weaknesses are a) the lack of consideration and measurement of GABAergic mechanisms of sevoflurane anesthesia, b) the failure to use another anesthetic as a control, c) a failure to document a compelling post-anesthesia stress response to sevoflurane in humans, d) limitations in the novelty of the findings. These weaknesses are related to the primary concerns described below:
Concerns about the primary conclusion, that PVH-CRH neurons mediate "the anesthetic effects and post-anesthesia stress response of sevoflurane GA".
Thanks for the advice. Our responses are as below:
- Just because the activity of a given neural cell type or neural circuit alters an anesthetic's response, this does not mean that those neurons play a role in how the anesthetic creates its anesthetic state. For example, sevoflurane is commonly used in children. Its primary mechanism of action is through enhancement of GABA-mediated inhibition. Children with ADHD on Ritalin (a dopamine reuptake inhibitor) who take it on the day of surgery can often require increased doses of sevoflurane to achieve the appropriate anesthetic state. The mesocortical pathway through which Ritalin acts is not part of the mechanism of action of sevoflurane. Through this pathway, Ritalin is simply increasing cortical excitability making it more challenging for the inhibitory effects of sevoflurane at GABAergic synapses to be effective. Similarly, here, altering the activity of the PVHCRH neurons and seeing a change in anesthetic response to sevoflurane does not mean that these neurons play a role in the fundamental mechanism of this anesthetic's action. With the current data set, the primary conclusions should be tempered.
Thank you for your comments. Our results adequately uncover PVH CRH neurons that modulate the state of consciousness as well as the stress response in sevoflurane GA, but are insufficient to demonstrate that these neurons play a role in the underlying mechanism of sevoflurane anesthesia. We will revise our conclusions and make them concrete. The primary conclusion has been revised as PVH CRH neurons potently modulate states of anaesthesia in sevoflurane GA, being a part of the anaesthesia regulatory network of sevoflurane.
- It is important to compare the effects of sevoflurane with at least one other inhaled ether anesthetic. Isoflurane, desflurane, and enflurane are ether anesthetics that are very similar to each other, as well as being similar to sevoflurane. It is important to distinguish whether the effects of sevoflurane pertain to other anesthetics, or, alternatively, relate to unique idiosyncratic properties of this gas that may not be a part of its anesthetic properties.
For example, one study cited by the authors (Marana et al.. 2013) concludes that there is weak evidence for differences in stress-related hormones between sevoflurane and desflurane, with lower levels of cortisol and ACTH observed during the desflurane intraoperative period. It is not clear that this difference in some stress-related hormones is modeled by post-sevoflurane excess grooming in the mice, but using desflurane as a control could help determine this.
Thank you for your suggestions. We completely agree on the importance of determining whether the effects of sevoflurane apply to other anesthetics or arise from unique idiosyncratic attributes separate from its anesthetic properties. However, it is challenging to definitively conclude whether the effects of sevoflurane observed in our study extend to other inhaled anesthetics, even with desflurane as a control. While sevoflurane shares many common anesthetic properties with other inhalation agents, it also exhibits distinct characteristics and potential idiosyncrasies that set it apart from its counterparts. Regarding studies related to desflurane's impact on hormone levels or stress-like behaviors, one study involving 20 women scheduled for elective total abdominal hysterectomy demonstrated that there was no significant correlation between the intra-operative depth of anesthesia achieved with desflurane and the extent of the endocrine-metabolic stress response (as indicated by the concentrations of plasma cortisol, glucose, and lactate)1. Besides, a study conducted with mice suggested the abilities related to sensorimotor functions, anxiety and depression did not undergo significant changes after 7 days of anesthesia administered with 8.0% desflurane for 6 h2. Furthermore, a study involving 50 Caucasian women undergoing laparoscopic surgery for benign ovarian cysts demonstrated that in low stress surgery, desflurane, when compared to sevoflurane, exhibited superior control over the intraoperative cortisol and ACTH response 3. Based on these findings, we propose that the effect we observed in this study is likely attributed to the unique idiosyncratic properties of sevoflurane. We will conduct additional experiments to investigate this proposal with other commonly used anaesthetics in our future studies.
Concerns about the clinical relevance of the experiments
In anesthesiology practice, perioperative stress observed in patients is more commonly related to the trauma of the surgical intervention, with inadequate levels of antinociception or unconsciousness intraoperatively and/or poor post-operative pain control. The authors seem to be suggesting that the anesthetic itself is causing stress, but there is no evidence of this from human patients cited. We were not aware that this is a documented clinical phenomenon. It is important to know whether sevoflurane effectively produces behavioral stress in the recovery room in patients that could be related to the putative stress response (excess grooming) observed in mice. For example, in surgeries or procedures that required only a brief period of unconsciousness that could be achieved by administering sevoflurane alone (comparable to the 30 min administered to the mice), is there clinical evidence of post-operative stress?
Thank you for your question. There is currently no direct evidence available. Studies on sevoflurane in humans primarily focus on its use during surgical interventions, making it difficult to find studies that solely administer sevoflurane, as was done in our study with mice. Generally, a short anesthesia time refers to procedures that last less than one hour, while a long anesthesia time could be considered for procedures lasting several hours or more4. A study published in eLife investigated the patterns of reemerging consciousness and cognitive function in 30 healthy adults who underwent GA for three hours 5. This finding suggests that the cognitive dysfunction observed immediately and persistently after GA in healthy animals may not necessarily apply anesthesia and postoperative neurocognitive disorders could be influenced by factors other than GA, such as surgery or patient comorbidity. Therefore, further studies are needed to verify the post-operative stress in sevoflurane-only short time anesthesia.
Indeed, stress after surgeries can result from multiple factors aside from anesthesia, including pain, anxiety, inflammation, but what we want to illustrate in this study is that anesthesia could be one of these factors that we ignored in previous studies. In our current study, we did not propose that there was a direct relationship between sevoflurane anesthesia and sevoflurane-mediated stress without incision. We observed stress-related behavioural changes after exposure of sevoflurane GA in mouse model, indicating sevoflurane-mediated stress might exist without surgical trauma. Importantly, whether anesthetic administration alone will cause post-operative stress is worth studying in different species especially human.
Patients who receive sevoflurane as the primary anesthetic do not wake up more stressed than if they had had one of the other GABAergic anesthetics. If there were signs of stress upon emergence (increased heart rate, blood pressure, thrashing movements) from general anesthesia, the anesthesiologist would treat this right away. The most likely cause of post-operative stress behaviors in humans is probably inadequate anti-nociception during the procedure, which translates into inadequate post-op analgesia and likely delirium. It is the case that children receiving sevoflurane do have a higher likelihood of post-operative delirium. Perhaps the authors' studies address a mechanism for delirium associated with sevoflurane, but this is not considered. Delirium seems likely to be the closest clinical phenomenon to what was studied.
We agree with your idea. We aim to establish a connection between post-operative delirium in humans and stress-like behaviors observed in mice following sevoflurane anesthesia. Specifically, we have observed that the increased grooming behavior exhibited by mice after sevoflurane anesthesia resembles the fuzzy state of consciousness experienced during post-operative delirium6. In our discussion, we also emphasized the occurrence of sevoflurane-induced emergence agitation, a common phenomenon reported in clinical studies with an incidence of up to 80%. This state is characterized by hyperactivity, confusion, delirium, and emotional agitation 7,8. Meanwhile, in our experimental tests, namely the open field test (OFT) and elevated plus maze (EPM) test, we observed that mice exposed to sevoflurane inhalation displayed reduced movement distances during both the OFT and EPM tests (Figure 7G and I). These findings suggest a decline in behavioral activity similar to what is observed in cases of delirium.
Concerns about the novelty of the findings
CRH is associated with arousal in numerous studies. In fact, the authors' own work, published in eLife in 2021, showed that stimulating the hypothalamic CRH cells leads to arousal and their inhibition promotes hypersomnia. In both papers, the authors use fos expression in CRH cells during a specific event to implicate the cells, then manipulate them and measure EEG responses. In the previous work, the cells were active during wakefulness; here- they were active in the awake state that follows anesthesia (Figure 1). Thus, the findings in the current work are incremental.
Thank you for acknowledging our previous work focusing on the changes in the sleep-wake state of mice when PVH CRH neurons are manipulated. In this study, our primary objective was to identify the neuronal mechanisms mediating the anesthetic effects and post-anesthetic stress response of sevoflurane GA. While our study claims that activation of PVH CRH neurons leads to arousal, it provides evidence that PVH CRH neurons may play a role in the regulation of conscious states in GA. Our current findings uncover that PVH CRH neurons modulate the state of consciousness as well as the stress response in sevoflurane GA, and that the modulation of PVH CRH neurons bidirectionally altered the induction and recovery of sevoflurane GA. This identifies a new brain region involved in sevoflurane GA that goes beyond the arousal-related regions.
The activation of CRH cells in PVN has already been shown to result in grooming by Jaideep Bains (cited as reference 58). Thus, the involvement of these cells in this behavior is expected. The authors perform elaborate manipulations of CRH cells and numerous analyses of grooming and related behaviors. For example, they compare grooming and paw licking after anesthesia with those after other stressors such as forced swim, spraying mice with water, physical attack, and restraint. However, the relevance of these behaviors to humans and generalization to other types of anesthetics is not clear.
The hyperactivity of PVH CRH neurons and behavior (e.g., excessive self-grooming) in mice may partially mirror the observed agitation and underlying mechanisms during emergence from sevoflurane GA in patients. As mentioned in the Discussion section (page 16, lines 371-374), sevoflurane-induced emergence agitation represents a prevalent manifestation of the post-anesthesia stress response. It is frequently observed, with an incidence of up to 80% in clinical reports, and is characterized by hyperactivity, confusion, delirium, and emotional agitation7,8. Our aim in this study is to distinguish the excessive stress responses of patients to sevoflurane GA from stress triggered by other factors. Other stimuli, such as forced swimming, can be considered sources of both physical and emotional stress, which are associated with depression and anxiety in humans.
Regarding generalization to other types of anesthetics, we propose that the stress-related behavioral effects observed in this study might occur in cases of the administration of certain types of anesthetics. For example, one study showed that intravenous ketamine infusion (10 mg/kg, 2 hours) elevated plasma corticosterone and progesterone levels in rats, reducing locomotor activity (sedation) 9. The administration of intravenous anesthesia with propofol combined with sevoflurane caused greater postoperative stress than the single use of propofol10. However, desflurane, a common inhaled ether anesthetic, when compared to sevoflurane, was associated with better control of intraoperative cortisol and ACTH response in low-stress surgeries8. Thus, these behaviors observed after exposure to sevoflurane GA may be related to the post-anesthesia stress response in humans, which might also occur in cases of the administration of certain types of anesthetics.
Recommendations for the authors:
Reviewer 1
- The CRH-Cre mouse line should be validated. There are several lines of these mice, and their fidelity varies.
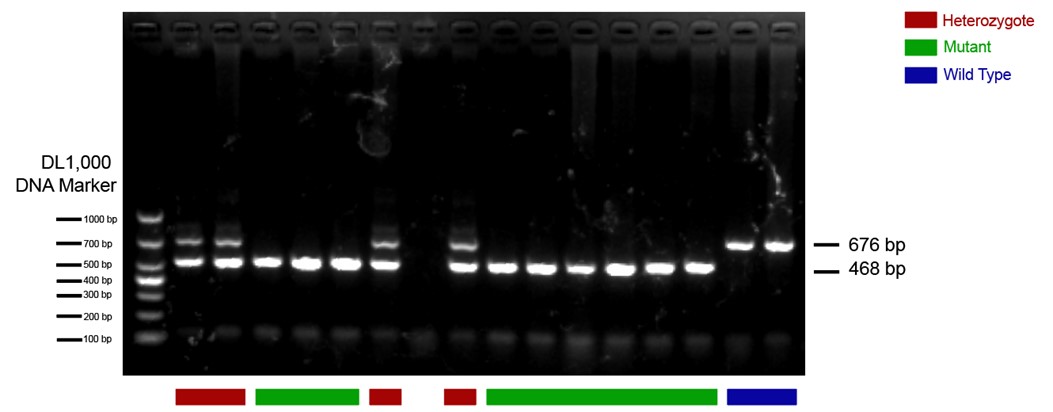
The CRH-Cre mouse line we used in this study is from The Jackson Laboratory (https://www.jax.org/strain/012704) with the name B6(Cg)-Crhtm1(cre)Zjh/J (Strain #: 012704). These CRH-ires-CRE knock-in mice have Cre recombinase expression directed to CRH positive neurons by the endogenous promoter/enhancer elements of the corticotropin releasing hormone locus (Crh). We have done standard PCR to validate the mouse line following genotyping protocols provided by the Jackson Laboratory. The protocol primers were: 10574 (SEQUENCE 5' → 3': CTT ACA CAT TTC GTC CTA GCC); 10575 (SEQUENCE 5' → 3': CAC GAC CAG GCT GCG GCT AAC); 10576 (SEQUENCE 5' → 3': CAA TGT ATC TTA TCA TGT CTG GAT CC). The 468-bp CRH-specific PCR product was amplified in mutant (CRH-Cre+/+) mice; in heterozygote (CRH-Cre+/-) mice, both the 468-bp and the 676-bp PCR products were detected; in wild type (WT) mice, only the 676-bp WT allele-specific PCR product was amplified. An example of PCR results is presented below. The heterozygote and mutant mice were included in our study.
Author response image 1.
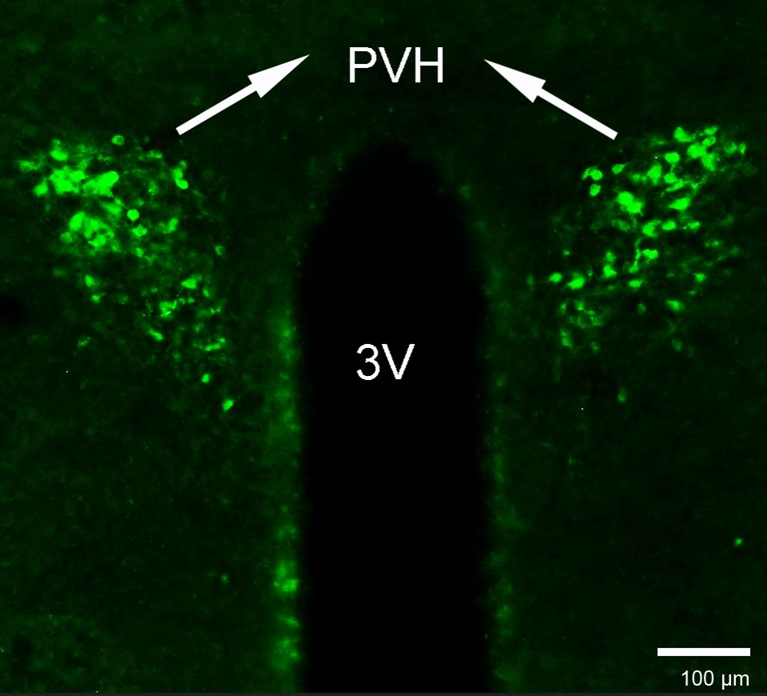
- It would be very helpful to validate the CRH antibody. Using any antiserum at 1:800 suggests that it may not be potent or highly specific.
As requested, we used the same CRH antibody at a concentration of 1:800, following the methods described in the Method section. The results are displayed below.
Author response image 2.
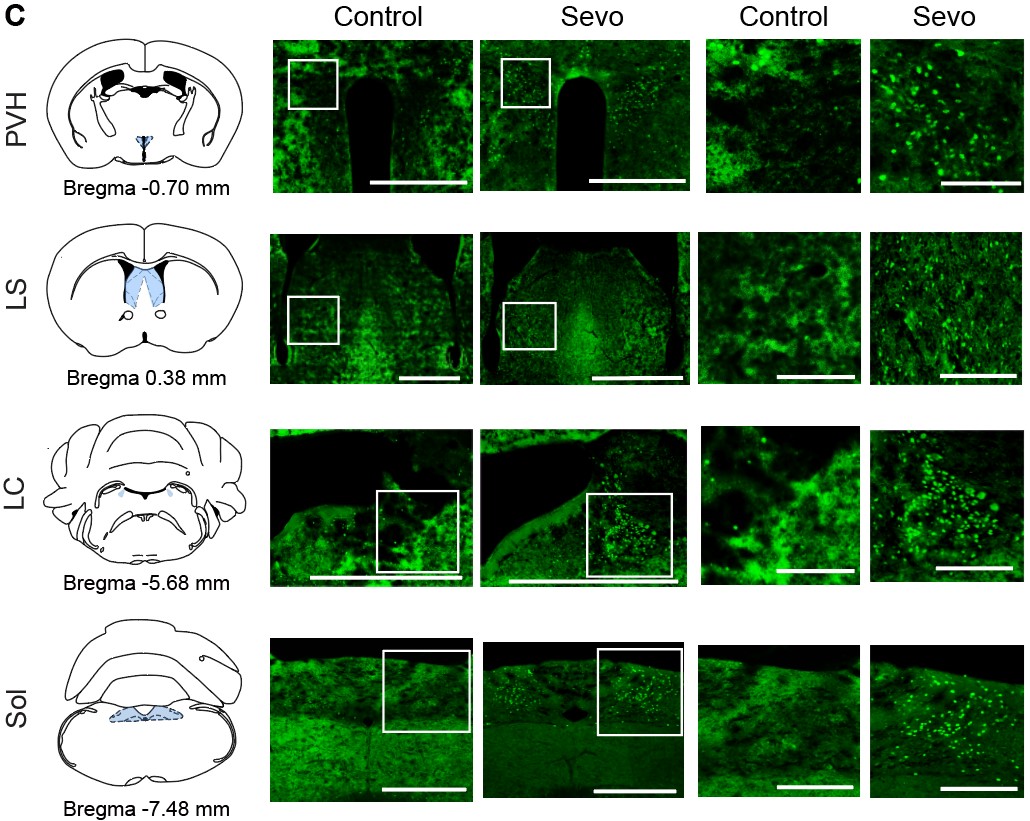
- In Figure 1C, the control sections are out of focus, any cells are blurry, reducing confidence in the analyses (locus ceruleus cells appear confluent in the control?)
Sorry for the confusing figure and we have revised the control section part of Figure 1C:
Author response image 3.
Reviewer 2
- In the Abstract, to say that "General anesthetics benefit patients undergoing surgeries without consciousness. ..." is a gross understatement of the essential role that general anesthesia plays today to make surgery not only tolerable but humane. This opening sentence should be rewritten. General anesthesia is a fundamental process required to undertake safely and humanely a high fraction of surgeries and invasive diagnostic procedures.
As requested, we rewrote this opening sentence, please see the follows:
GA is a fundamental process required to undertake surgeries and invasive diagnostic procedures safely and humanely. However, the undesired stress response associated with GA can lead to delayed recovery and even increased morbidity in clinical settings.
- In the Abstract, when discussing the response of the PVN-CRH neurons to chemogenetic inhibition, say exactly what the "opposite effect" is.
Thanks for your insights. We have rewritten our abstract as follows:
Chemogenetic activation of these neurons delayed the induction and accelerated emergence from sevoflurane GA, whereas chemogenetic inhibition of PVH CRH neurons promoted induction and prolonged emergence from sevoflurane GA.
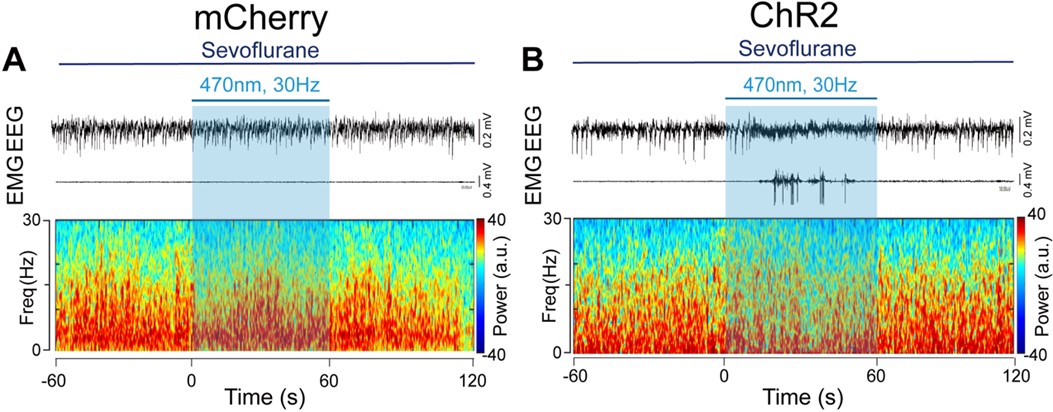
- In all spectrograms the dynamic range is compressed between 0.5 and 1. Please make use of the full range, as some details might be missed because of this compression.
We are sorry for the incorrect unit of the spectrograms. We have provided the correct one with full range, please see below:
Author response image 4.
Author response image 5.
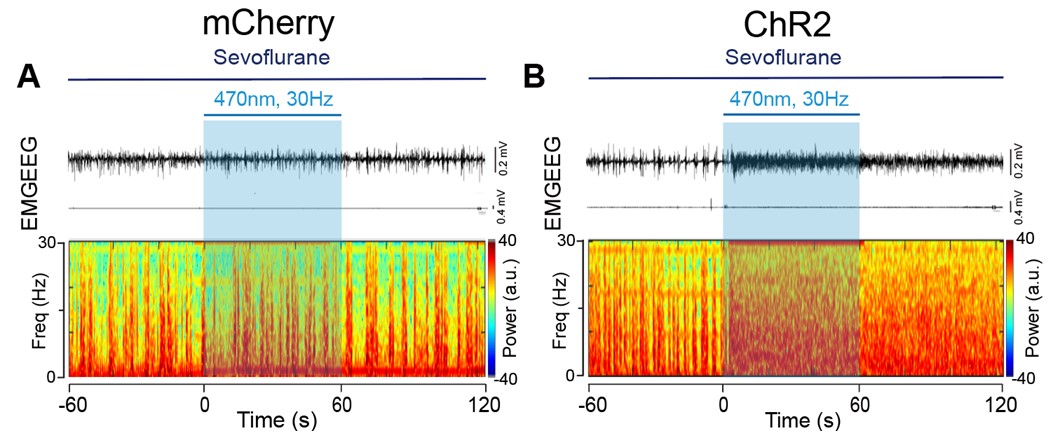
- The spectrogram in Figure 2D has several frequency chirps that do not seem physiological.
Thank you for your comments. The frequency chips of the spectrogram during the During and Post 1 phase were caused by recording noises. To avoid confusion, we have deleted the spectrogram in Figure 2D.
- The 3D plots in Figures 3G and H are not helpful.
Thanks for the comment. We'd like to keep the 3D plots as they aid visual comparison of three different features of grooming, which complements other panels in Figure 3.
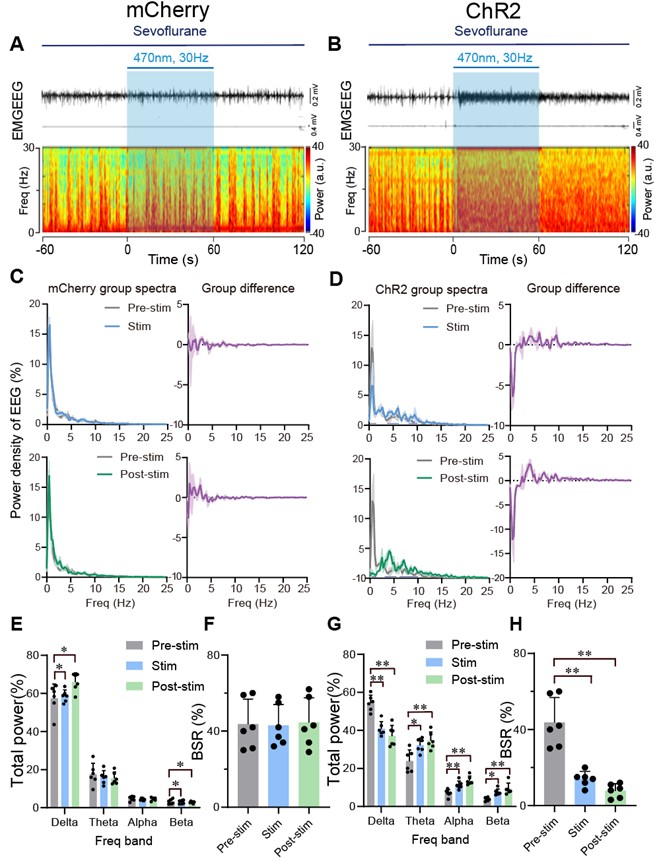
- The spectrograms in Figures 5A and B are too small, while the spectra in Figures 5C and D are too large. Please invert this relationship, as it is interesting and important to see the details in the spectrograms. The same happens in Figure 6.
We adjusted the layout of the Figure 5 and Figure 6 as requested, please see below:
Author response image 6.
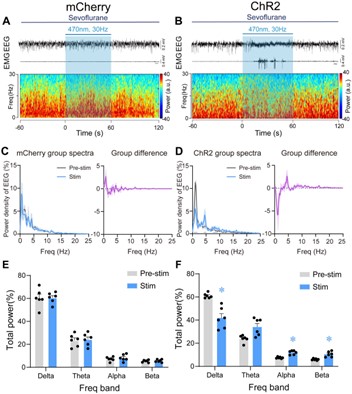
Author response image 7.
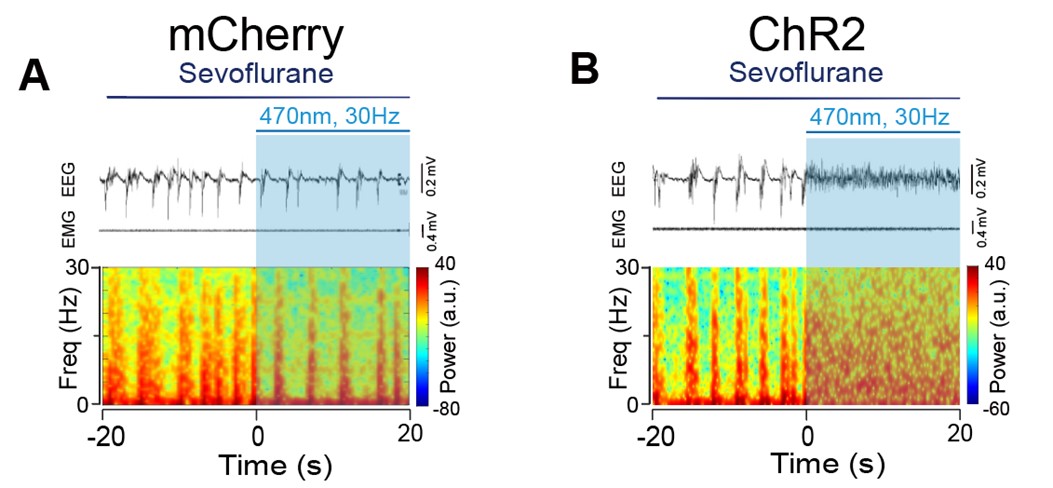
- In Figure 6H, the authors compute the burst-suppression ratio during a period that seemingly has no bursts or suppressions (Figure 6B).
The burst-suppression ratio was computed from data with the minimum duration of burst and suppression periods set at 0.5 s. Sorry for the confusion. We added a new supplementary figure (Figure 6-figure supplement 8) displaying a 40-second EEG with a burst suppression period to better visualize the burst suppression.
Author response image 8.
- The data analyses are done in terms of p-values. They should be reported as confidence intervals so that any effect the authors wish to establish is measured along with its uncertainty.
Thank you for your valuable suggestions regarding our manuscript. We appreciate your thoughtful consideration of our work. We understand your concern but we would like to provide some justification for our choice of reporting p-values and explain why we believe they are appropriate for our study. First, the use of p-values for hypothesis testing and significance assessment is a common practice in our field. Many previous studies in our area of research also report results in terms of p-values. For example, Wei Xu11 published in 2020 suggested sevoflurane inhibits MPB neurons through postsynaptic GABAA-Rs and background potassium channels, Ao Y12 demonstrated that activation of the TH:LC-PVT projections is helpful in facilitating the transition from isoflurane anesthesia to an arousal state, using P-value as data analyses. By adhering to this convention, we ensure that our findings are consistent with the existing body of literature. This makes it easier for readers to compare and integrate our results with previous work. Secondly, while confidence intervals can provide a measure of effect size and uncertainty, p-values offer a concise way to communicate statistical significance. They help readers quickly assess whether an effect is statistically significant or not, which is often the primary concern when interpreting research findings. We hope that by providing these reasons for our choice of reporting p-values, we can address your concern while maintaining the integrity and consistency of our study. If you believe there are specific instances where reporting confidence intervals would be more informative, please feel free to highlight those, and we will consider your suggestion on a case-by-case basis.
References
- Baldini, G., Bagry, H. & Carli, F. Depth of anesthesia with desflurane does not influence the endocrine-metabolic response to pelvic surgery. Acta Anaesthesiol Scand 52, 99-105, doi:10.1111/j.1399-6576.2007.01470.x (2008).
- Niikura, R. et al. Exploratory analyses of postanesthetic effects of desflurane using behavioral test battery of mice. Behav Pharmacol 31, 597-609, doi:10.1097/fbp.0000000000000567 (2020).
- Marana, E. et al. Desflurane versus sevoflurane: a comparison on stress response. Minerva Anestesiol 79, 7-14 (2013).
- Vutskits, L. & Xie, Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 17, 705-717, doi:10.1038/nrn.2016.128 (2016).
- Mashour, G. A. et al. Recovery of consciousness and cognition after general anesthesia in humans. Elife 10, doi:10.7554/eLife.59525 (2021).
- Mattison, M. L. P. Delirium. Ann Intern Med 173, Itc49-itc64, doi:10.7326/aitc202010060 (2020).
- Dahmani, S. et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 104, 216-223, doi:10.1093/bja/aep376 (2010).
- Lim, B. G. et al. Comparison of the incidence of emergence agitation and emergence times between desflurane and sevoflurane anesthesia in children: A systematic review and meta-analysis. Medicine (Baltimore) 95, e4927, doi:10.1097/MD.0000000000004927 (2016).
- Radford, K. D. et al. Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats. Behav Brain Res 378, 112259, doi:10.1016/j.bbr.2019.112259 (2020).
- Yang, L., Chen, Z. & Xiang, D. Effects of intravenous anesthesia with sevoflurane combined with propofol on intraoperative hemodynamics, postoperative stress disorder and cognitive function in elderly patients undergoing laparoscopic surgery. Pak J Med Sci 38, 1938-1944, doi:10.12669/pjms.38.7.5763 (2022).
- Xu, W. et al. Sevoflurane depresses neurons in the medial parabrachial nucleus by potentiating postsynaptic GABA(A) receptors and background potassium channels. Neuropharmacology 181, 108249, doi:10.1016/j.neuropharm.2020.108249 (2020).
- Ao, Y. et al. Locus Coeruleus to Paraventricular Thalamus Projections Facilitate Emergence From Isoflurane Anesthesia in Mice. Front Pharmacol 12, 643172, doi:10.3389/fphar.2021.643172 (2021).











