Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorJuan Alvaro GallegoImperial College London, London, United Kingdom
- Senior EditorYanchao BiPeking University, Beijing, China
Reviewer #1 (Public review):
This study extends the previous interesting work of this group to address the potentially differential control of movement and posture. Their earlier work explored a broad range of data to make the case for a downstream neural integrator hypothesized to convert descending velocity movement commands into postural holding commands. Included in that data were observations from people with hemiparesis due to stroke. The current study uses similar data, but pushes into a different, but closely related direction, suggesting that these data may address the independence of these two fundamental components of motor control. I find the logic laid out in the second sentence of the abstract ("The paretic arm after stroke is notable for abnormalities both at rest and during movement, thus it provides an opportunity to address the relationships between control of reaching, stopping, and stabilizing") less then compelling, but the study does make some interesting observations. Foremost among them, is the relation between the resting force postural bias and the effect of force perturbations during the target hold periods, but not during movement. While this interesting observation is consistent with the central mechanism the authors suggest, it seems hard to me to rule out other mechanisms, including peripheral ones. These limitations should should be discussed.
Reviewer #2 (Public review):
Summary:
Here the authors address the idea that postural and movement control are differentially impacted with stroke. Specifically, they examined whether resting postural forces influenced several metrics of sensorimotor control (e.g., initial reach angle, maximum lateral hand deviation following a perturbation, etc.) during movement or posture. The authors found that resting postural forces influenced control only following the posture perturbation for the paretic arm of stroke patients, but not during movement. They also found that resting postural forces were greater when the arm was unsupported, which correlated with abnormal synergies (as assessed by the Fugl-Meyer). The authors suggest that these findings can be explained by the idea that the neural circuitry associated with posture is relatively more impacted by stroke than the neural circuitry associated with movement. They also propose a conceptual model that differentially weights the reticulospinal tract (RST) and corticospinal tract (CST) to explain greater relative impairments with posture control relative to movement control, due to abnormal synergies, in those with stroke.
Comments on revisions:
The authors should be commended for being very responsive to comments and providing several further requested analyses, which have improved the paper. However, there is still some outstanding issues that make it difficult to fully support the provided interpretation.
The authors say within the response, "We would also like to stress that these perturbations were not designed so that responses are directly compared to each other ***(though of course there is an *indirect* comparison in the sense that we show influence of biases in one type of perturbation but not the other)***." They then state in the first paragraph of the discussion that "Remarkably, these resting postural force biases did not seem to have a detectable effect upon any component of active reaching but only emerged during the control of holding still after the movement ended. The results suggest a dissociation between the control of movement and posture." The main issue here is relying on indirect comparisons (i.e., significant in one situation but not the other), instead of relying on direct comparisons. Using well-known example, just because one group / condition might display a significant linear relationship (i.e., slope_1 > 0) and another group / condition does not (slope_2 = 0), does not necessarily mean that the two groups / conditions are statistically different from one another [see Figure 1 in Makin, T. R., & Orban de Xivry, J. J. (2019). Ten common statistical mistakes to watch out for when writing or reviewing a manuscript. eLife, 8, e48175.].
The authors have provided reasonable rationale of why they chose certain perturbation waveforms for different. Yet it still holds that these different waveforms would likely yield very different muscular responses making it difficult to interpret the results and this remains a limitation. From the paper it is unknown how these different perturbations would differentially influence a variety of classic neuromuscular responses, including short-range stiffness and stretch reflexes, which would be at play here.
Much of the results can be interpreted when one considers classic neuromuscular physiology. In Experiment 1, differences in resting postural bias in supported versus unsupported conditions can readily be explained since there is greater muscle activity in the unsupported condition that leads to greater muscle stiffness to resist mechanical perturbations (Rack, P. M., & Westbury, D. R. (1974). The short-range stiffness of active mammalian muscle and its effect on mechanical properties. The Journal of physiology, 240(2), 331-350.). Likewise muscle stiffness would scale with changes in muscle contraction with synergies. Importantly for experiment 2, muscle stiffness is reduced during movement (Rack and Westbury, 1974) which may explain why resting postural biases do not seem to be impacting movement. Likewise, muscle spindle activity is shown to scale with extrafusal muscle fiber activity and forces acting through the tendon (Blum, K. P., Campbell, K. S., Horslen, B. C., Nardelli, P., Housley, S. N., Cope, T. C., & Ting, L. H. (2020). Diverse and complex muscle spindle afferent firing properties emerge from multiscale muscle mechanics. eLife, 9, e55177.). The concern here is that the authors have not sufficiently considered muscle neurophysiology, how that might relate to their findings, and how that might impact their interpretation. Given the differences in perturbations and muscle states at different phases, the concern is that it is not possible to disentangle whether the results are due to classic neurophysiology, the hypothesis they propose, or both. Can the authors please comment.
The authors should provide a limitations paragraph. They should address 1) how they used different perturbation force profiles, 2) the muscles were in different states which would change neuromuscular responses between trial phase / condition, 3) discuss a lack of direct statistical comparisons that support their hypothesis, and 4) provide a couple of paragraphs on classic neurophysiology, such as muscle stiffness and stretch reflexes, and how these various factors could influence the findings (i.e., whether they can disentangle whether the reported results are due to classic neurophysiology, the hypothesis they propose, or both).
Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
This study extends the previous interesting work of this group to address the potentially differential control of movement and posture. Their earlier work explored a broad range of data to make the case for a downstream neural integrator hypothesized to convert descending velocity movement commands into postural holding commands. Included in that data were observations from people with hemiparesis due to stroke. The current study uses similar data but pushes into a different, but closely related direction, suggesting that these data may address the independence of these two fundamental components of motor control. I find the logic laid out in the second sentence of the abstract ("The paretic arm after stroke is notable for abnormalities both at rest and during movement, thus it provides an opportunity to address the relationships between control of reaching, stopping, and stabilizing") less than compelling, but the study does make some interesting observations. Foremost among them, is the relation between the resting force postural bias and the effect of force perturbations during the target hold periods, but not during movement. While this interesting observation is consistent with the central mechanism the authors suggest, it seems hard to me to rule out other mechanisms, including peripheral ones.
Response 1.1. Thank you for your comments, which we address in detail below and in our response to Recommendations to the authors (see pp. 15-19 of this letter). We would first like to clarify the motivation behind our use of a stroke population to understand the interactions between the control of reaching in and holding. We agree that this idea can be laid out in a more compelling way.
The fact that stroke patients usually display issues with their control of both reaching and holding, allows for within-individual comparisons of those two modes of control. Further, the magnitude of abnormalities is relatively large, making it easier to measure, compare and investigate effects. And, importantly, these two modes of control can be differentially affected after stroke (also pointed out by Reviewer 2, point 4 in Comments to the Authors). Finally, this kind of work – examining interactions between positive signs of stroke (such as abnormal posture or synergy) vs. negative signs (such as loss of motor control) – needs to be done in humans, as positive signs are relatively absent even in primates (Tower, 1940).
We have changed our abstract (changes shown below in red), and our intro (expanding the second paragraph, lines 75-76), to lay out our motivation more clearly.
From the abstract:
“The paretic arm after stroke exhibits different abnormalities during rest vs. movement, providing an opportunity to ask whether control of these behaviors is independently affected in stroke. “
On the other hand, the relation between force bias and the well-recognized flexor synergy seems rather self-evident, and I don't see that these results add much to that story.
Response 1.2. While it seems natural that these biases would be the resting expression of abnormal flexor synergies (given their directionality towards the body, as shown in Figures 2-3, and the other similarities we demonstrate in Figure 8), we do not believe it is self-evident. These biases are measured at rest, with the patient passively moved and held still, whereas abnormal synergies emerge when the patient actively tries to move. The lack of relationship we find between these resting force biases and active movement underlines that the relation between force bias and flexor synergy should not be taken as self-evident, making it worthwhile to examine it (as we motivate in lines 589-596 and show in Figure 8).
The paradox here is that, in spite of a relationship between force bias and flexor synergy (itself manifesting during attempted movement), there seems to be no relationship between force bias and direct measures of active movement (Figures 5,6). This is the paradox that inspired our conceptual model (Figure 9) and inspires to further investigate the factors under which these two systems are intermingled or kept separate. We thus find it to be a helpful element in the story.
I am also struck by what seems to be a contradiction between the conclusions of the current and former studies: "These findings in stroke suggest that moving and holding still are functionally separable modes of control" and "the commands that hold the arm and finger at a target location depend on the mathematical integration of the commands that moved the limb to that location." The former study is mentioned here only in passing, in a single phrase in the discussion, with no consideration of the relation between the two studies. This is odd and should be addressed.
Response 1.3. While these two sets of findings are not contradictory, we understand how they can appear as such without providing context. We now discuss the relationship between our present study and the previous one more directly (lines 66-70 and 663-669 of the revised manuscript).
The previous study examined how the control of movement informs the control of holding after the movement was over; the current study examines whether abnormalities in holding measured at rest with the movement leading to the rest position being passive. There are thus two important distinctions:
First, directionality of potential effects: here we examine the effect of (abnormalities in) holding control upon movement, but the 2020 study (Albert et al., 2020) examines the effects of movement upon holding control. Stroke patient data in the 2020 study showed that, under CST damage, while the reach controller is disrupted, the hold controller can continue to integrate the malformed reach commands faithfully. In line with this, we proposed a model where the postural controller system sits downstream of the moving controller (Figure 7G in the 2020 paper). We thus did not claim, in 2020, that integration of movement commands is the only way to do determine posture control, as we stated explicitly back then, e.g. (emphasis ours):
“Equations (1) and (2) describe how the integration of move activity may relate to changes in hold commands, but does not specify the hold command at the target.”
In short, finding no effect of holding abnormalities upon movement (present finding) does not mean there is no potential effect of movement upon holding (2020 finding). This is something we had alluded to in the Discussion but not clarified, which we do now (see edits at the end of our response to this point).
Second, active vs. passive movement: here, we measure holding control at rest (Experiment 1). The 2020 study shows that endpoint forces reflect the integration of learned dynamics exerted during active movement that led to the endpoint position. However, in Experiment 1, there is no active reaching to integrate, as the robot passively moves the arm to the held position. Thus, resting postural forces measured in Experiment 1 could not reflect the integration of reach commands that led to each rest position.
Thus, the two sets of findings are not contradictory. Taking our current and 2020 findings together suggests that active holding control would comprise would reflect both the integration of movement control that led to assuming the held position, plus the force biases measured at rest.
Hence our decision to describe these two systems as functionally separable: while these systems can interact, the effects of post-stroke malfunctions in each can be independent depending on the function and conditions at hand. This does not make this a limited finding: being able to dissociate post-stroke impairment based on each of these two modes of control may inform rehabilitation, and also importantly, understanding the conditions in which these two modes of control become separable can substantially advance our understanding of both how different stroke signs interact with each other and how motor control is assembled in the healthy motor system. Figure 9 illustrates our conceptual model behind this and may serve as a blueprint to further dissect these circuits in the future.
We discuss these issues briefly in lines 663-669 in our Discussion section, reproduced below for convenience:
“It should be noted, however, that having distinct neural circuits for reaching and holding does not rule out interactions between them. For example, we recently demonstrated how arm holding control reflects the integration of motor commands driving the preceding active movement that led to the hold position, in both healthy participants and patients with hemiparesis (Albert et al., 2020). However, in that paper, we did not claim that this integration is the only source of holding control. Indeed, in Experiment 1 of the current study, we used passive movement to bring the arm to each probed position, which means that the postural biases could not be the result of integration of motor commands.”
And, we have adjusted our Introduction to provide pertinent context regarding our 2020 work (first paragraph, lines 66-70 of the updated manuscript).
A minor wording concern I had is that the term "holding still" is frequently hard to parse. A couple of examples: "These findings in stroke suggest that moving and holding still are functionally separable modes of control." This example is easily read, "moving and holding [continue to be] functionally separable". Another: "...active reaching and holding still in the same workspace, " could be "...active reaching and holding [are] still in the same workspace." Simply "holding", "posture" or "posture maintenance" would all be better options.
Response 1.4. Thank you for your suggestion. Following your comment, we have abbreviated this term to simply “holding”, both on the title and throughout the text.
Reviewer #2 (Public Review):
Summary:
Here the authors address the idea that postural and movement control are differentially impacted with stroke. Specifically, they examined whether resting postural forces influenced several metrics of sensorimotor control (e.g., initial reach angle, maximum lateral hand deviation following a perturbation, etc.) during movement or posture. The authors found that resting postural forces influenced control only following the posture perturbation for the paretic arm of stroke patients, but not during movement. They also found that resting postural forces were greater when the arm was unsupported, which correlated with abnormal synergies (as assessed by the Fugl-Meyer). The authors suggest that these findings can be explained by the idea that the neural circuitry associated with posture is relatively more impacted by stroke than the neural circuitry associated with movement. They also propose a conceptual model that differentially weights the reticulospinal tract (RST) and corticospinal tract (CST) to explain greater relative impairments with posture control relative to movement control, due to abnormal synergies, in those with stroke.
Strengths:
The strength of the paper is that they clearly demonstrate with the posture task (i.e., active holding against a load) that the resting postural forces influence subsequent control (i.e., the path to stabilize, time to stabilize, max. deviation) following a sudden perturbation (i.e., suddenly removal of the load). Further, they can explain their findings with a conceptual model, which is depicted in Figure 9.
Weaknesses:
Current weaknesses and potential concerns relate to i) not displaying or reporting the results of healthy controls and non-paretic arm in Experiment 2 and ii) large differences in force perturbation waveforms between movement (sudden onset) and posture (sudden release), which could potentially influence the results and or interpretation.
Response 2.0. Thank you for your assessment, and for pointing out ways to improve our paper. We address the weakness and potential concerns in detail below.
Larger concerns
(1) Additional analyses to further support the interpretation. In Experiment 1 the authors present the results for the paretic arm, non-paretic arm, and controls. However, in Experiment 2 for several key analyses, they only report summary statistics for the paretic arm (Figure 5D-I; Figure 6D-E; Figure 7F). It is understood that the controls have much smaller resting postural force biases, but they are still present (Figure 3B). It would strengthen the position of the paper to show that controls and the non-paretic arm are not influenced by resting postural force biases during movement and particularly during posture, while acknowledging the caveat that the resting positional forces are smaller in these groups. It is recommended that the authors report and display the results shown in Figure 5D-I; Figure 6D-E; Figure 7F for the controls and non-paretic arm. If these results are all null, the authors could alternatively place these results in an additional supplementary.
Response 2.1a. Thank you for your recommendations. We agree both on the value of these analyses and the caveat associated with them: these resting postural force biases are substantially smaller for the non-paretic and control data (for example, the magnitude of resting biases in the supported condition is 2.8±0.4N for the paretic data, but only 1.8±0.4N and 1.3±0.2N for the non-paretic and control data, respectively; the difference is even greater in the unsupported condition, though this is not the one being compared to Experiment 2).
We now conduct a comprehensive series of supplementary analyses, including the examination of non-paretic and control data for all three components of Experiment 2 (unperturbed reaches; pulse perturbations; and active holding control). These are mentioned in the Results (lines 422-424, 512513, and 574-574 of the revised manuscript) and illustrated in the supplementary materials: Supplementary Figures S5-1, S6-1, and S7-1 contain the main analyses (comparisons of instances with the most extreme resting biases for each individual) for the unperturbed reach analysis, pulse perturbation analysis, and active holding control analysis, respectively.
We find that non-paretic and control data do not display effects of resting biases upon unperturbed reaching control (Figure S5-1) or control against a pulse perturbation early during movement (Figure S6-1) – as is the case with the paretic data. Non-paretic and control data do not display evidence of influence of their resting force biases upon active holding control either (Figure S7-1), unlike the paretic data. For the non-paretic data, however, these influences are nominally towards the same direction as in the paretic data. Given that resting biases are substantially weaker for the non-paretic case, it is possible a similar relationship exists but requires increased statistical power to discern. Moreover, it is possible that the effect of resting biases is non-linear, with small biases effectively kept under check so that their impact upon active holding control is even less than a linearly scaled version of the impact of the stronger, paretic-side biases. This can be the subject of future work.
Please also note that, following your recommendation (Recommendations to the Authors, point 2.1), we have conducted secondary analyses which estimate sensitivity to resting bias using all datapoints, validating our main analyses; these analyses were also performed for control and non-paretic data, with similar results (Response 2.A.1).
Further, the results could be further boosted by reporting/displaying additional analyses. In Figure 6D the authors performed a correlation analysis. Can they also display the same analysis for initial deviation and endpoint deviation for the data shown in Figure 5D-F & 5G-I, as well for 7F for the path to stabilization, time to stabilization, and max deviation? This will also create consistency in the analyses performed for each dependent variable across the paper.
Response 2.1b. Here, we set to test whether resting biases affect movement. It is best to do this using a within-individual comparison design, rather than using across-individual correlations: while correlation analyses can in general be informative, they obscure within-individual effects which are the main comparisons of interest in our study. Consider a participant with strong resting bias towards one direction, tested on opposing perturbations; averaging these responses for each individual would mostly cancel out any effects of resting biases. Even if we were to align responses to the direction of the perturbation before averaging, the power of correlation analyses may be diluted by inter-individual differences in other factors, such as overall stiffness.
Thus, our analysis design was instead focused on examining the differential effects of resting posture biases within each individual’s data. We compared the most extreme opposing/aligned or clockwise/counter-clockwise instances within each individual, specifically to assess these differential effects. In our revised version, we have further reinforced these analyses to include all data rather than the most extreme instances (see response 2.A.1.a to the Reviewer’s recommendation to the authors) where we performed correlations of within-individual resting posture vs. the corresponding dependent variables and compared the resulting slopes.
The across-individual correlation analyses add little to that for the reasons we outlined above. At the same time, it is possible they can be helpful in e.g. illustrating across-individual variability. We thus now include across-individual correlation analyses for all dependent variables, but, given their limited value, only in the supplementary material. This also means that, for consistency, we moved the correlation analysis in Figure 6 to the corresponding supplementary figure as well (Figure S6-3).
In addition, following the Reviewer’s comment about consistency in the analyses performed for each dependent variable across the paper, we added within-individual comparisons for settling time following the pulse perturbations (Figure 6D, right).
(2) Inconsistency in perturbations that would differentially impact muscle and limb states during movement and posture. It is well known that differences in muscle state (activation / preloaded, muscle fiber length and velocity) and limb state (position and velocity) impact sensorimotor control (Pruszynski, J. A., & Scott, S. H. (2012). Experimental brain research, 218, 341-359.). Of course, it is appreciated that it is not possible to completely control all states when comparing movement and posture (i.e., muscle and limb velocity). However, using different perturbations differentially impacts muscle and limb states. Within this paper, the authors used very different force waveforms for movement perturbations (i.e., 12 N peak, bell-shaped, 0.7ms duration -> sudden force onset to push the limb; Figure 6A) and posture perturbations (i.e., 6N, 2s ramp up -> 3s hold -> sudden force release that resulted in limb movement; Figure 4) that would differentially impact muscle (and limb) states. Preloaded muscle (as in the posture perturbation) has a very different response compared to muscle that has little preload (as in the movement perturbations, where muscles that would resist a sudden lateral perturbation would likely be less activated since they are not contributing to the forward movement). Would the results hold if the same perturbation had been used for both posture and movement (e.g., 12 N pulse for both experiments)? It is recommended that the authors comment and discuss in the paper why they chose different perturbations and how that might impact the results.
Response 2.2a. We agree that it can be impossible to completely control all states when comparing movement and posture. We would also like to stress that these perturbations were not designed so that responses are directly compared to each other (though of course there is an indirect comparison in the sense that we show influence of biases in one type of perturbation but not the other). Instead, Experiment 2 tried to implement a probe optimized for each motor control modality (moving vs. holding). However, the Reviewer has a point that the potential impact of differences between the perturbations is important to discuss in the paper.
The Reviewer points out two potentially interesting differences between the two perturbations. First, the magnitude (6N for the posture perturbation vs. 12N for the pulse perturbation); second, the presence of background load in the posture perturbation, in contrast to the pulse perturbation.
For the movement perturbation, we used a 12-N, 70ms pulse. This perturbation and scaled versions have been tested before in both control and patient populations (Smith et al., 2000; Fine and Thoroughman, 2006). For the holding perturbation, we used a background load to ensure that active holding control is engaged, and the duration of the probe (holding for about 5s) made using a stronger perturbation impractical –maintaining a background load at, say, 12N for that long could lead to increased fatigue.
The question raised by the Reviewer, whether the findings would be the same if the same, 12-N pulse were used to probe both moving and holding control, is interesting to investigate. We would expect the same qualitative findings (i.e. there would still be a connection between resting posture and active holding control when the latter were probed with a 12N pulse). Recent work provides more specific insight into what to expect. Our posture perturbation task is similar to the Unload Task in (Lowrey et al., 2019), whereby a background torque is released, whereas our pulse perturbation is more similar to their Load Task, whereby a torque is imposed against no background load (though it is a step perturbation rather than a pulse). Lowrey et al., 2019 find that their Unload task is harder than the Load task, with 2x the fraction of patient trials classified as failed (with failure defined as task performance being outside of the 95% confidence interval for controls), though there are still clear effects for the Load task.
This suggests that the potential effects of using a pulse-like perturbation to probe posture control would likely be weaker in magnitude, all other things being equal. At the same time, however, the Load and Unload tasks in Lowrey et al., 2019 were perturbations of the same magnitude; it is thus also likely that the reduction in effect would be mitigated, or reversed, by the fact that we would be using a 12N instead of a 6N perturbation.
A relevant consequence of the Lowrey et al., 2019 findings is that the Unload paradigm is superior in its ability to detect impairment in static, posture perturbations, and thus provides a better signal to detect potential relationships with resting posture biases. This is not surprising, as a background load further engages the control of active holding, which what we were trying to probe in the first place.
But then why not use the same paradigm (preloading and release) for movement? There are two main reasons. First, requiring a background load throughout the experiment is unfeasible due to fatigue. Second, for the holding perturbation, we wanted to ensure that the postural control system is meaningfully engaged when the perturbation hits, hence we picked the background load. Were we to impose the same during moving – i.e. impose a lateral background load on the movement - we could be engaging posture control on top of movement control. This preloading would reduce the degree to which the pulse probe isolates movement control, and lead to intrusion of the posture control system in the movement task by design. This relates to what the Reviewer proposes in the comment below: preloading may result in postural biases i.e. engage posture control; see below where we argue this interpretation is within the scope of our conceptual model rather a counter to it.
We now explain the rationale behind our perturbation design in the Methods section (lines 211-220).
Relatedly, an alternative interpretation of the results is that preloading muscle for stroke patients, whether by supporting the weight of one's arm (experiment 1) or statically resisting a load prior to force release (experiment 2), leads to a greater postural force bias that can subsequently influence control. It is recommended that the authors comment on this.
Response 2.2b. We find this interpretation valid, but we do not see how it meaningfully differs from the framework we propose. We already state that the RST may be tailored for both posture/holding control and the production of large forces (which would include muscle preloading):
“Thus, the accumulated evidence suggests that the RST could control posture and large force production in the upper limb.“ (lines 698-699 in the current version)
“the RST, in contrast, is weighted more towards slower postural control and generation of large isometric forces” (lines 724-726 in the current version)
And, we discuss other conditions where the RST is involved in large force production, such as power grip, and how these interact with the role of the RST in posture/holding control (lines 758-768 in the current version).
To better explain our model, we now provide the two examples mentioned by the reviewer along with our description of the proposed role for the RST (lines 726-727):
“…the RST, in contrast, is weighted more towards slower postural control and generation of large isometric forces (such as vertical forces for arm support, or horizontal forces for holding the arm still against a background load like in our posture/release perturbation trials).”
We note, however, that we find resting posture abnormalities even in the presence of arm support, suggesting the involvement of the RST in holding control even when the forces involved (and the need to preload the muscle) are small.
Reviewer #3 (Public Review):
The authors attempt to dissociate differences in resting vs active vs perturbed movement biases in people with motor deficits resulting from stroke. The analysis of movement utilizes techniques that are similar to previous motor control in both humans and non-human primates, to assess impairments related to sensorimotor injuries. In this regard, the authors provide additional support to the extensive literature describing movement abnormalities in patients with hemiparesis both at rest and during active movement. The authors describe their intention to separate out the contribution of holding still at a position vs active movement as a demonstration that these two aspects of motor control are controlled by two separate control regimes.
Strengths:
(1) The authors utilize a device that is the same or similar to devices previously used to investigate motor control of movement in normal and impaired conditions in humans and non-human primates. This allows comparisons to existing motor control studies.
(2) Experiment 1 demonstrates resting flexion biases both in supported and unsupported forelimb conditions. These biases show a correlated relationship with FM-UE scores, suggesting that the degree of motor impairment and the degree of resting bias are related.
(3) The stroke patient participant population had a wide range of both levels of impairment and time since stroke, including both sub-acute and chronic cases allowing the results to be compared across impairment levels.
The authors describe several results from their study: 1. Postural biases were systematically toward the body (flexion) and increased with distance from the body (when the arm was more extended) and were stronger when the arm was unsupported. 2. These postural biases were correlated with FM-UE score. 3. They found no evidence of postural biases impacting movement, even when that movement was perturbed. 4. When holding a position at the end of a movement, if the position was perturbed opposite of the direction of bias, movement back to the target was improved compared to the perturbation in the direction of bias. Taken together, the authors suggest that there are at least two separate motor controls for tasks at rest versus with motion. Further, the authors propose that these results indicate that there is an imbalance between cortical control of movement (through the corticospinal tracts) and postural control (through the reticulospinal tract).
Response 3.1. Thank you for pointing out some of the strengths of our work and summarizing our findings. A minor clarification we would like to make, related to (3), is that, while our study did enroll two patients towards the end of the subacute stage (2-3 months), the rest of the population were at the chronic stage, at one year and beyond. We thus find it very unlikely that time after stroke was the primary driver of differences in impairment in the population we studied.
There are several weaknesses related to the interpretation of the results:
In Experiment 1, the participants are instructed to keep their limbs in a passive position after being moved. The authors show that, in the impaired limb, these resting biases are significantly higher when the limb is unsupported and increase when the arm is moved to a more extended position.
When supported by the air sled, the arm is in a purely passive position, not requiring the same antigravity response so will have less RST but also less CST involvement. While the unsupported task invokes more involvement of the reticulospinal tract (RST), it likely also has significantly higher CST involvement due to the increased difficulty and novelty of the task.
If there were an imbalance in CST regulating RST as proposed by the authors, the bias should be higher in the supported condition as there should be relatively less CST activation/involvement/ modulation leading to less moderating input onto the RST and introducing postural biases. In the unsupported condition, there is likely more CST involvement, potentially leading to an increased modulatory effect on RST. If the proportion of CST involvement significantly outweighs the RST activation in the unsupported task, then it isn't obvious that there is a clear differentiation of motor control. As the degree of resting force bias and FM-UE score are correlated, an argument could be made that they are both measuring the impairment of the CST unrelated to any RST output. If it is purely the balance of CST integrity compared to RST, then the degree of bias should have been the same in both conditions. In this idea of controller vs modulator, it is unclear when this switch occurs or how to weigh individual contributions of CST vs. extrapyramidal tracts. Further, it isn't clear why less modulation on the RST would lead only to abnormal flexion.
Response 3.2. Our model posits two mechanisms by which CST impairment would lead to increased RST involvement. The first – which is the one discussed by the Reviewer here - is a direct one, whereby weaker modulation of the RST by the CST leads to increased RST involvement. The second is an indirect one, whereby the incapacity of CST to drive sufficient motor output to deal with tasks eventually leads to increased RST drive.
The reviewer suggests it is likely that the unsupported task demands increased activation through both the CST and the RST. If that were the case, however, it would exaggerate the effects of CST/RST imbalance after stroke compared to healthy motor control: if task conditions (lack of support) required higher CST involvement, then CST damage would have an even larger effect. In turn, this would lead to even higher RST involvement and further diminishing the ability of CST to moderate RST. Thus, RST-driven biases would be higher in the unsupported condition.
And, given that the CST itself is damaged and has to deal with an even-increased RST activation, we would not expect that the proportion of CST involvement would outweigh RST activation, but the opposite. In fact, a series of relatively recent findings suggest just this. For example,
• Zaaimi et al., 2012 showed that unilateral CST lesions in monkeys lead to significant increases in the excitability of the contralesional RST (Zaaimi et al., 2012). Interestingly, this effect was present in flexors but not extensors, potentially explaining why less modulation and/or overactivation of the RST would primarily lead to abnormal flexion.
• McPherson et al. (further discussed in point 2.A.23, by Reviewer 2 – Recommendations to the Authors) showed that, after stroke, contralesional activity (which would include the ipsilateral RST) increases relative to ipsilesional activity (which would include the contralateral CST)
(McPherson et al., 2018). The same study also provides evidence that FM-UE may primarily reflect RST-driven impairment. The ipsilateral(RST)/contralateral(CST) balance, expressed as a laterality index, correlated with FM-UE, with lower FM-UE for indices indicating higher RST involvement. (Interestingly, the slope of this relationship was steeper when the laterality of brain activation patterns was examined under tasks with less arm support, mirroring the steeper FM-UE vs resting bias slope when arm support is absent, as shown in our Figure 8).
• Wilkins et al., 2020 (Wilkins et al., 2020) found that providing less support (i.e. requiring increased shoulder abduction) increases ipsilateral activation (representing RST) relative to contralateral activation (representing CST).
This resting bias could be explained by an imbalance in the activation of flexors vs extensors which follows the results that this bias is larger as the arm is extended further, and/or in a disconnect in sensory integration that is overcome during active movement. Neither would necessitate separate motor control for holding vs active movement.
Response 3.3. We do not think that either of these points necessarily argue against our model. First, the resting biases we observe are clearly pointed towards increased flexion, and can thus be seen as the outcome of an imbalance in the activation of flexors vs. extensors at rest. This imbalance between flexors/extensors can also be explained by the CST/RST imbalance posited by our conceptual model: in their study of CST lesions in the monkey, Zaaimi et al., 2012 found increased RST activation for flexors but not extensors, suggesting that RST over-involvement may specifically lead to flexor abnormalities (Zaaimi et al., 2012). Second, overcoming a disconnect in sensory integration may be one way the motor system switches between separate controllers; how this switch happens is not examined by our conceptual model.
In Experiment 2, the participants are actively moving to and holding at targets for all trials while being supported by the air sled. Even with the support, the paretic participants all showed start- and endpoint force biases around the movement despite not showing systematic deviations in force direction during active movement start or stop. There could be several factors that limit systematic deviations in force direction. The most obvious is that the measured biases are significantly higher when the limb is unsupported and by testing with a supported limb the authors are artificially limiting any effect of the bias.
Response 3.4. We do expect, in line with what the reviewer suggests, that any potential effects would be stronger in the unsupported condition. The decision to test active motor control with arm support was done as running the same Experiment 2 would pose challenges, particularly with our most impaired patients, given the duration of Experiment 2 (~2 hours, about 1 hour with each arm) and the expected fatigue that would ensue.
However, a key characteristic of our comparisons is that we are comparing Experiment 2 active control data under arm support, against Experiment 1 resting bias data also under arm support. While Experiment 1 measured biases without arm support as well, these are not used for this comparison. And, while resting biases are weaker with arm support, they are still clear and significant; yet they do not lead to detectable changes in active movement.
At the same time, we do not rule out that, if we were to repeat Experiment 2 without arm support, we could find some systematic deviation in the direction of resting bias in movement control. Our conceptual model, in fact, suggests that this may be the case, as we described in lines 618-620 of our original manuscript. The idea here is that, when arm support is not provided, the increased strength requirements lead to increased drive through the RST, to the point that posture control (and its abnormalities) spills into movement control (Figure 9). We now better clarify this position in our Discussion (lines 744-750):
“The interesting implication of this conceptual model is that synergies are in fact postural abnormalities that spill over into active movement when the CST can no longer modulate the increased RST activation that occurs when weight support is removed (i.e. resting biases may influence active reaching in absence of weight support). Supporting this idea, a study found increased ipsilateral activity (which primarily represents activation via the descending ipsilateral RST (Zaaimi et al., 2012)) when the paretic arm had reduced support compared to full support (McPherson et al., 2018).”
It is also possible that significant adaptation or plasticity with the CST or rubrospinal tracts could give rise to motor output that already accounts for any intrinsic resting bias.
Response 3.5. This kind of adaptation – regardless of the tracts potentially involved – is an issue we examined in our experiment. As we talk about in our Results (lines 458-460 in the updated manuscript), with most of our patient population in the chronic stage, it could be likely that their motor system adapted to those biases to the point that movement planning took them into account, thereby limiting their effect. This motivated us to examine responses to unpredictable perturbations during movement (Figure 6) where we still find lack of an obvious effect of resting biases upon reaching control. We thus believe that our findings are not explained by this kind of adaptation, though we agree it would be of great interest for future work to compare resting biases and reaching control in acute vs. chronic stroke populations to examine the degree to which stroke patients adapt to these biases as they recover.
In any case, the results from the reaching phase of Experiment 2 do not definitively show that directional biases are not present during active reaching, just that the authors were unable to detect them with their design. The authors do acknowledge the limitations in this design (a 2D constrained task) in explaining motor impairment in 3D unconstrained tasks.
Response 3.6. It is, of course, an inherent limitation of a negative finding is that it cannot be proven. What we show here is that, there is no hint of intrusion of resting posture abnormalities upon active movement in spite of these resting posture abnormalities being substantial and clearly demonstrated even under arm support. To allow for the maximum bandwidth to detect any such effects, we specifically chose to compare the most extreme instances (resting bias-wise) for each individual, and yet we did not find any relationship between biases and active reaching.
This suggests that, even if these biases could be in some form present during active movement, their effect would be minimal and thus limited in meaningfully explaining post-stroke impairment in active movement under arm support.
Note that, as we already discuss, our conceptual model (Figure 9) suggests that the degree to which directional biases would be present in active reaching may be influenced by arm support (or the specific movements examined – hence our limitation in not examining 3D movement). Thus we do not claim that this independence is absolute. Examples include the last line of the passage quoted right above, and the summary statement of our Discussion quoted below (lines 639-641):
“…which raises the possibility that the observed dissociation of movement and posture control for planar weight-supported movements may break down for unsupported 3D arm movements.”
Finally, we now more explicitly acknowledge that abnormal resting biases may influence active movement in the absence of arm support (see Response 3.4).
It would have been useful, in Experiment 2, to use FM-UE scores (and time from injury) as a factor to determine the relationship between movement and rest biases. Using a GLMM would have allowed a similar comparison to Experiment 1 of how impairment level is related to static perturbation responses. While not a surrogate for imaging tractography data showing a degree of CST involvement in stroke, FM-UE may serve as an appropriate proxy so that this perturbation at hold responses may be put into context relative to impairment.
Response 3.7. Here the Reviewer suggests we use FM-UE scores as a proxy for CST integrity. We do not think this analysis would be particularly helpful in our case for a number of reasons:
First, while FM-UE is a general measure of post-stroke impairment, it was designed to track - among other things - the emergence and resolution of abnormal synergies, a sign assumed to result from abnormally high RST outflow (McPherson et al., 2018; McPherson and Dewald, 2022). In line with this, the FM-UE scales with EMG-based measures of synergy abnormality (Bourbonnais et al., 1989). Impairments in dexterity, a sign associated with damage to the CST (Lawrence and Kuypers, 1968; Porter and Lemon, 1995; Duque et al., 2003), dissociate with synergy abnormalities when compared under arm support as we do here (Levin, 1996; Hadjiosif et al., 2022). This means that FM-UE would be a stronger proxy for RST activity and thus not a direct proxy for CST integrity particularly when one wants to dissociate RST-specific vs. CST-specific abnormalities. In fact, as we discuss in Response 3.2 above, there is a number of studies supporting this idea: for example, Zaaimi et al., 2012 show that relative RST activation – the balance between ipsilateral excitability, primarily reflecting RST, and contralateral excitability, primarily reflecting the CST, scales with FM-UE (Zaaimi et al., 2012).
Second, this kind of analysis would obscure within-individual effects, since FM-UE scores are, of course, assigned to each individual. This is the same issue as doing across-individual correlation analyses in general (see response 2.1b).Strong resting force bias would have opposite effects on opposing perturbations, averaging across subjects would occlude these effects.
Third, while FM-UE is a good measure of synergy abnormality, weakness alone could also give an abnormal FM-UE (Avni et al., 2024).
The Reviewer also suggests we use time from injury for this analysis. Time from injury can indeed potentially be an important factor. However, this analysis would not be appropriate for our dataset, since the effective variation in recovery stage within our population is limited: our sample is essentially chronic (only two patients were examined within the subacute stage – at 2 and 3 months after stroke - with everybody else examined more than a year after stroke) with the “positive” elements of their phenotype (and FM-UE itself) essentially plateaued (Twitchell, 1951; Cortes et al., 2017). We thus would not expect to see any meaningful effects of time from injury within our population. It would be an excellent question for future work to investigate both resting biases and their relationship to reaching in acute/subacute patients, and examine whether the trajectory of resting biases (both emergence and abatement due to recovery) follows the one for abnormal synergies.
It is not clear that even in the static perturbation trials that the hold (and subsequent move from perturbation) is being driven by reticulospinal projections. Given a task where ~20% of the trials are going to be perturbed, there is likely a significant amount of anticipatory or preparatory signaling from the CST. How does this balance with any proposed contribution that the RST may have with increased grip?
Response 3.8. We included our response to this as part of Response 3.2. In brief, while we cannot rule out that these tasks may recruit increased CST signaling, this would tend to increase, rather than reduce, the effects of post-stroke impairment: the requirement for increased signaling from a CST that is damaged would magnify the effects of this damage, in turn leading to increased recruitment of other tracts, such as the RST.
In general, the weakness of the interpretation of the results with respect to the CST/RST framework is that it is necessary to ascribe relative contributions of different tracts to different phases of movement and hold using limited or indirect measures. Barring any quantification of this data during these tasks, different investigators are likely to assess these contributions in different ways and proportions limiting the framework's utility.
Response 3.9. We believe that our Reponses 3.2-3.6 put our findings in fair perspective, and the edits undertaken based on the Reviewer’s comments have clarified our position as to how the dissociation between holding and moving control may break down. We do agree, however, that our framework would be strengthened by the use of direct measures of CST/RST connectivity in future research. We present our conceptual model as a comprehensive explanation of our findings and how they blend with current hypotheses regarding the role of these two tracts in motor control after stroke. As such, it provides a blueprint towards future research that more directly measures or modulates CST and RST involvement, using tools such as tractography or non-invasive brain stimulation.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
L226 “…of this issue, we repeated the analysis of Figure 7F (a) by excluding these four patients…”. Should this be three, based on the previous sentence?
Response 1.A.1. Thank you for pointing this typo, which is now corrected. The analysis in question (Figure S1 in the original submission, now re-numbered as Figure S7-4), excluded the three patients mentioned in the previous sentence.
L254 “…the hand was held in a more distal position. The postural force biases were strongest when…” Could this be "extended" rather than distal? See my later comment about the inadequate description of targets.
Response 1.A.2. The reviewer is correct that, the arm will tend to be more extended in the distal targets. However, since these positions were defined in extrinsic coordinates, we think the terms distal/proximal are also appropriate. In either case, we now clarify these definitions in the text (see Response 1.A.3 below).
L263 “…contained both distal and proximal targets, and, importantly, they were also the movement…”. Distal/proximal targets were never described as part of the task.
Response 1.A.3. We improved our description by (i) changing the wording above to “represented positions both distal and proximal to the body,”, (ii) doing the same in our Methods (line 175) and (iii) indicating distal/proximal targets in Figure 3A (bottom right of panel A).
L378 “…the pulse perturbation. We hypothesized that, should resting postural forces play a role, they…” L379 “…would tend to reduce the effect of the pulse if they were in the opposite direction, and…” Not really obvious why. A reduction in the displacement caused by a force pulse might be caused by different stiffness or viscosity, but not by a linear, time-invariant force bias. This situation is different from that of "moving the arm through a high-postural bias area vs. a low-postural bias area" where it would encounter time- (actually spatially) varying forces and varying amounts of displacement. Clarify the logic if this is a critical point.
Response 1.A.4. We thank the Reviewer for highlighting this point of potential confusion. We now clarify that these postural bias forces are neuromuscular in origin (Kanade-Mehta et al., 2023), and likely result from an expression of abnormal synergy, at least under static conditions. In this case, we hypothesized that force pulses acting against the gradient of the postural bias field would act to stretch the already active muscles, which would lead to a further increase in postural resistance due to inherent length-tension properties of active muscle. By contrast, force pulses acting along the gradient of the postural bias field would act to shorten the same active muscles, which would lead to a reduction in postural resistance. The data did not support this in the case of force pulses imposed during movement. We note, however, that similar effects would affect responses to static perturbations as well, wherein we do find an effect of resting biases. We now better explain this reasoning (lines 479482).
L466 “resting postural force). In short, our perturbations revealed that resting flexor biases switched 467 on after movement was over, providing evidence for separate control between moving” and
L468 “holding still.”
I do not think the authors have presented clear evidence that forces, "switch on", implying the switch to a different controller which they posit. This could as easily be a nonlinear or time-varying property of a single controller (admittedly, the latter possibility overlaps broadly with their idea of distinct, interacting controllers). An example that the authors are certainly aware of is that of muscle "thixotropy" a purely peripheral mechanism due to the dynamics of crossbridge cycling that causes resting muscle to be stiffer than moving muscle, changing with a time constant of ~1-2 seconds. Neither this particular example nor changing levels of contraction (more likely during the unpredictable force perturbations) would be in the direction to explain the main observation here -- a point perhaps worth making, together with the stretch reflex comments.
Response 1.A.5. Thank you for this perspective. Indeed, it might be that “switching on” represents a shift along a nonlinear property of the same controller: in the extreme, if this nonlinearity is a step (on/off) function, this single controller would be functionally identical to two separate controllers. We thus cannot tell if these controllers are distinct in the strict sense. What we argue here is that, no matter the underlying controller architecture - two distinct controllers or two distinct modes of the same controller - is that the control of reaching vs. holding can be functionally separable even after stroke. In line with this idea, we used a more nuanced phrasing (e.g. “separable functional modes for moving vs. holding”) throughout our manuscript, and we have now edited out a mention of “separate controllers” to be consistent with this.
Moreover, thank you for pointing out the example of thixotropy, showing how peripheral mechanisms could interact with central control. As you point out, this effect would not explain the main observation here: in fact, if stiffness were substantially higher during rest or holding (instead of moving) that would reduce the impact of the static perturbation, making it harder to detect any effects of resting biases compared to the moving perturbation case.
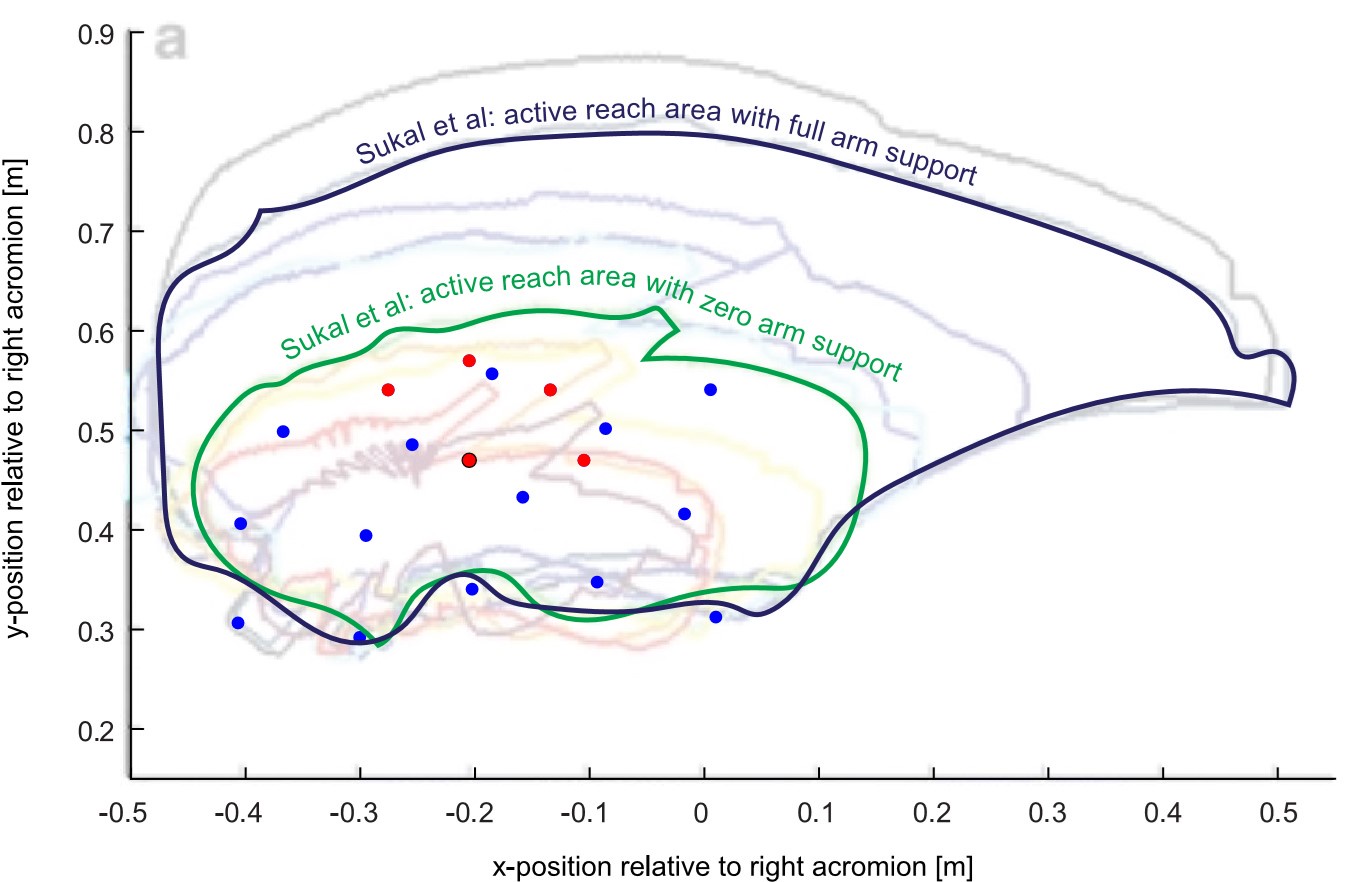
L480 “…during movement (Sukal et al., 2007). Yet, Experiment 2 found no relationship between resting…” L481”… postural force biases and active movement control. To further investigate this apparent…” The methods of the two studies seem fairly similar, but this question warrants a more careful comparison. How did the size of the two workspaces compare? What about the magnitude of the exerted forces? The movement condition in this study was done with the limb entirely supported. Under that condition, the Sukal study also found fairly small effects of the range of motion.
Response 1.A.6. Sukal et al., 2007 did not directly measure exerted forces, but instead compared the active range of motion under different loading conditions. They used the extent of reach area to quantify the effect of abnormal synergies, with a more extended active range of motion signifying reduced effect of abnormal synergies. As the Reviewer points out, Sukal et al. found fairly small effects of synergies upon the range of motion when arm support was provided (the reach area for the paretic side was found to be about 85% of the nonparetic side under full arm support, though they were statistically significantly different, Figure 5 of their paper). They found increasing effect of synergies as arm support was reduced: on average, the reach area when participants had to fully support the arm was less than 50% the reach area when full arm support was given (comparing the 0% vs. 100% active support conditions [i.e. 100% vs. 0% external support] in their Figure 5). As we discuss in our paper, this effect of arm support upon synergy mirrors the one we found for resting postures.
To compare our workspace with the one in Sukal et al., we overlaid our workspace (the array of positions for which the posture biases were measured, for a typical participant from Experiment 1) on the one they used as shown in their Figure 4. Note that their figure only shows an example participant, and thus our ability to compare is limited by the fact that each participant can vary widely in terms of their impairment, and assumptions had to be made to prepare this overlay (e.g. that (0,0) represents the position of the right acromion point).
For this example, and our assumptions, our workspace was smaller, with the main points of interest (red dots, the movement start/end points used for Experiment 2) within the Sukal et al. workspace. That our workspace is smaller is not surprising, given that the area in Sukal et al. represents the limit of what can be reached, and thus motor control *has* to be examined in a subset of that area.
Author response image 1.
Comparing the two study methodologies, however, suggests an advantage of measuring resting biases in terms of sensitivity and granularity: first, resting biases can be clearly detected even under arm support (something we point out in our Discussion, lines 715-717); second, they can measure abnormalities at any point in the workspace, rather than a binary within/without the reach area. The resting bias approach may thus be a more potent tool to probe the shared bias/synergy mechanisms we propose here.
Figure 2
Needs color code.
The red dots could be bigger.
Response 1.A.7. We have increased the size of the red dots and added a color code to explain the levels illustrated by the contours. We also expanded our caption to better explain this illustration.
Figure 3
Labeling is confusing. Drop the colored words (from both A and B), and stick to the color legend. Consider using open and filled symbols (and bars) to represent arm support or lack thereof. The different colored ovals are very hard to distinguish.
Response 1.A.8. We find these recommendations improve the readability of Figure 3 and we have thus adopted them - see updated Figure 3.
Figure 4
Not terribly necessary.
Response 1.A.9. While this figure is indeed redundant based our descriptions in the text, we kept it as we believe it can be useful in clarifying the different stages of movement we examine.
Figure 5
Tiny blue and green arrows are impossible to distinguish.
Although the general idea is clear, E and H are not terribly intuitive. Add distance scale bars for D-I.
Response 1.A.10. For improved contrast, we now use red and blue (also in line with comment below regarding Figure 7), and switched to brighter colors in general. To make E and H more intuitive and easier to follow, we expanded the on-panel legend. Thank you for pointing out that distance scale bars are missing; we have now added them (panels EFHI).
Figure 6
Panel E inset is too small.
Response 1.A.11. We have now moved the inset to the right and enlarged it.
Figure 7
Green and blue colors are not good.
Response 1.A.12. For improved contrast, we now use red and blue.
Figure 8
Delete or move to supplement?
Response 1.A.13. We respectfully disagree. While the relationships on these data are also captured by the ANOVA, we believe these scatter plots offer a better overview of the relationships between force biases and FM-UE across different conditions.
Really minor
L113 “…participants' lower arm was supported using a custom-made air-sled (Figure 1C). Above the participant's…”
Response 1.A.14. We put the apostrophe after the s so to refer to participants in general (plural).
L117 ”…subject-produced forces on the handle were recorder using a 6-axis force transducer.” recorded
Response 1.A.14. Thank you for pointing out this error which we have now corrected.
L136 “…2013), Experiment 1 assessed resting postural forces by passively moving participants to>…” The experiment did not move the participant.
Response 1.A.15. We now fix this issue: “by having the robot passively move…”
L248 “…experiment blocks: two with each arm, with or without arm weight support (provided by an air experimental…”
Response 1.A.16. We have now corrected this.
L364 “…responses to mid-movement perturbations. In 1/3 of randomly selected reaching movements…” Obviously, you mean 1/3 of all movements: "One-third of the reaching movements were chosen randomly"
Response 1.A.17. We now clarify: “In 1/3 of reaching movements in Experiment 2, chosen randomly”. Also please note our response to Reviewer 2, point 10: we now report the exact number of trials for which each kind of perturbation was present.
L609 “Damage to the CST after stroke reduces its moderating influence upon the RST (Figure 9,…” "its" refers to the subject, "Damage", not "CST".
Response 1.A.18. We have changed this to “Post-stroke damage to the CST reduces the moderating influence the CST has upon the RST”.
Reviewer #2 (Recommendations For The Authors):
(1) Throughout, the authors cleverly selected the most opposed and most aligned resting postural force biases to perform a within-subject analysis. However, this approach excludes a lot of data. The authors could perform an additional within-subject analysis. For each participant they could correlate lateral resting posture force bias to each dependent variable, utilizing all the trials of a participant.
Response 2.A.1a. Thank you for your appreciating our analysis design, and suggesting additional analyses. We focused our within-subject analysis design on the most extreme instances, as we believe that this approach would offer the best opportunity to detect any potential effects of resting biases. We reasoned that, since resting biases tend to be relatively small for most locations in the workspace, taking all biases into account would inject a disproportionate amount of noise in our analysis, which would in turn diminish our ability to detect any potential relationships. This could be because small biases lead to small effects but also small biases may themselves be more likely to reflect measurement noise in the first place. Note that our study talks about separability of active reaching from resting abnormalities based on lack of relationships between the two. While one cannot definitely prove a negative, it is also important to take the approach that maximizes the ability to detect any such relationship if there were one. We believe taking the most extreme instances fulfills that role.
However, as the Reviewer points out, this approach also excludes a substantial amount of data. We agree that our findings could be further strengthened by exploring additional within-subject analyses that utilize all trials. Thus, following the reviewer’s suggestion, we estimated the sensitivity of each dependent variable to lateral resting posture force bias. Specifically, we estimated the slope of this relationship for each individual (separately for paretic and non-paretic data) using linear regression, and assessed whether the average slope is significant for each group (paretic data, non-paretic data, and control data).
This secondary analysis replicated our main findings: lack of relationship between posture biases and active reaching control (both for unperturbed and perturbed movement), and a significant relationship between posture biases and active holding control. In addition, in line with main point 2.1 by the reviewer, we performed the same analyses for non-paretic and control data. While there are no definitive conclusions to be made for these cases (as was likely, given that the resting force biases are smaller, as also pointed out by the Reviewer in 2.1) these data are worthy of discussion, with potentially interesting insights (for example, there are hints that the connection between resting biases and active holding control is present in the non-paretic arm as well, and may be explored in future research).
We have included these analyses in the supplementary materials, and we point to them in the main text. Specifically:
First, in line with our main analyses in Figure 5, we find no effect (the average slope is insignificant) for start and endpoint biases upon the corresponding reaching angles. This is now mentioned in lines 425-434 of the Results, and illustrated in Figure S5-2. There was a lack of effect for the non-paretic and control data as well.
Second, in line with our main analyses in Figure 6, we find no effect of start biases upon responses to the pulse (Figure S6-2, mentioned in lines 513-517 of the Results). As above, there was no effect of non-paretic or control data either.
And, finally, in line with our main analysis in Figure 7, we find an effect of resting biases upon performance for the static perturbation (Figure S7-2, mentioned in lines 578-586 of the Results). Interestingly, there is a suggestion that resting biases may affect static perturbation responses in the non-paretic data as well based on the relationship between posture bias and maximum deviation, but not the other two metrics. Given the lack of consistency of resting bias effects for all three different dependent variables examined, however, our current data are thus unable to give a definite answer as to whether there is the connection between resting biases and active holding control is also present in the non-paretic side. Our hypothesis is that, since resting abnormalities and their effects are the pathological over-manifestations of mechanisms inherent in the motor system in general, then such a relationship would exist. Answering this question, however, would require an experiment design better tailored to detect relationships in the non-paretic arm, where resting biases are weaker.
We thank the Reviewer for their suggestions and believe that these additional analyses provide a more complete picture of the data, and their consistency with our main results reinforces the message of the paper.
Then, they can report the percentage of participants that display significant correlations separately for the paretic, nonparetic, and control arms.
Response 2.A.1b. We note that, even in cases where the average slope (across individuals) is significant, the individual slopes themselves are usually not significant, likely due to the large amount of noise for datapoints corresponding to weak resting biases. To further examine this, we performed additional analyses whereby we examined slopes by (a) pooling all participant data together (centered separately for each individual), and then (b) took a further step to normalize each participant’s data not only by centering but by also adjusting by each individual’s variability along each axis (i.e. assess the slope between z-scores of resting bias vs. z-scores of each dependent variable). These two analyses confirmed our finding that resting biases interacted with active motor control, with significant slopes between resting biases and outcome variables. (a) Pooling all data together: path to stabilization: p = 0.032; time to stabilization: p = 1.4x10-5; maximum deviation: p = 0.021. (b) Pooling and normalizing: path to stabilization: p = 0.0013; time to stabilization: p = 8.6x10-6; maximum deviation: p = 0.00056. The latter analysis showed even stronger connection between resting bias and active holding control, probably due to better accounting for differences in the range of resting biases across participants). For simplicity, however, we only provide the across-individual slope comparisons in the paper.
(2) An important aspect of all the analyses is that they rely heavily on estimates of the resting postural force bias. How stable are these resting postural force biases at the individual level? The authors could assess this by reporting within-subject variance for both the magnitude and direction of the resting postural force bias.
Response 2.A.2. Thank you for your suggestion. We now assess the individual-level variance in error across measurements for patients’ paretic data using an ANOVA: the variance that remains after all other factors (same probe location; same arm support condition; same participant) are taken into account. We found that individual level measurement variance explained a mere 9.0% of total variance for resting bias magnitude. (We note that the same figure was 20.2% for the non-paretic data, in line with the weaker average biases which would be more susceptible to noise). We now note this in the Methods, as part of the new subsection “Stability of resting posture bias measurements in Experiment 1” (lines 266-273).
(3) Does resting postural force bias influence hand movement immediately following force release from the postural perturbation? This could be assessed before any volitional responses by examining the velocity of the hand during the first 50 ms following the postural perturbation.
Response 2.A.3. The influence seems fairly rapid, within the first 100ms as shown to the right. Here we plot hand deviation in the direction of the perturbation for the most-opposed (red) vs. most-aligned (blue) instances to examine when these curves become different. The bottom plots show the difference between these two, whereas shading indicates SEM (note that these curves are referenced to the average deviation in the last 0.5 s before force release). The rightmost plots zoom in to make it easier to see how responses to the most opposed vs. most aligned instances diverge.
To detect the earliest post-perturbation timepoint for which this effect was significant, we performed paired t-tests at each timestep, and found that the two responses were systematically statistically different 95ms after perturbation onset onwards. For reference, the same method detected a response at 25ms for the most aligned instances and 40ms for the most opposed instances.
We have now added Supplementary Figure S7-4 with short commentary in the Supplementary Materials.
(4) Abstract. lines 7-9. At a glance (and when reading the manuscript linearly) this sentence is unclear. If the paretic arm is compromised across rest and movement, how does that afford the opportunity to address the relationship between reaching, stopping, and stabilizing when all could be impacted? It might be useful to specify that these factors may impacted differently relative to one another with stroke, providing an opportunity to better understand the differences between movement and postural control.
Response 2.A.4. Thank you for pointing out this issue (also related to Reviewer 1’s point – Response 1.1). We have changed this to more clearly reflect our reasoning and highlight that the issue is that stroke can differentially impact reaching vs. holding, copied below:
“The paretic arm after stroke exhibits different abnormalities during rest vs. movement, providing an opportunity to ask whether control of these behaviors is independently affected in stroke.”
(5) Line 27. It is perhaps more appropriate to say conceptual model than simply 'model'.
Response 2.A.5. Thank you for your suggestion, which we have adopted throughout the manuscript.
(6) Line 122-125. Figure 1A caption. The authors should specify that resting posture force biases occur when the limb or hand is physically constrained in a specific position.
Response 2.A.6. Thank you for pointing this out – we have clarified the caption:
“If one were to physically constrain the hand in a position away from the resting posture, the torques involved in each component of the abnormal resting posture translate to a force on the hand (blue arrow);”
(7) Line 147. Why was the order not randomized or counterbalanced?
Response 2.A.7. We prioritized paretic data, as the primary analyses and comparisons in our paper involved resting posture biases and active movement with the paretic arm. We note that our primary analyses, which rely on paretic-paretic comparisons, would not be affected by paretic vs. non-paretic ordering effects. However, ordering effects could potentially affect comparisons between paretic and non-paretic data. We now note the reasoning behind the absence of counterbalancing, and mention the potential limitation in interpreting paretic to non-paretic comparisons in lines 124-129 of the Methods.
(8) Line 172. 12N is the peak force of the pulse?
Response 2.A.8. The reviewer is correct; we have clarified our description (line 463 in the updated manuscript):
“a 70 ms bell-shaped force pulse which was 12N at its peak”
(9) Line 175. What is a clockwise pulse? Was the force vector rotating in direction over time so that it was always acting orthogonally to the movement, or did it always act leftwards or rightwards?
Response 2.A.9. The force vector was not rotating in direction over time. Here, we used clockwise/counterclockwise to indicate rightwards/leftwards with respect to the ideal movement direction – the line from start position to target (which is what we understand the Reviewer means by “always act rightwards or leftwards”). We have clarified the text to indicate this (lines 193-195):
…was applied by the robot lateral to the ideal movement direction (i.e. the direction formed between the center of the start position and the center of the target) after participants reached 2cm away from the starting position (Smith and Shadmehr, 2005; Fine and Thoroughman, 2006).
(10) Lines 177-182. It might be useful to explicitly mention the frequency of each of the perturbations, just for ease of the reader.
Response 2.A.10. We have added this information to our Methods (lines 206-210):
Thus, in summary, each 96-movement block consisted of 64 unperturbed movements and 32 movements perturbed with a force pulse (16 clockwise, and 16 counter-clockwise). For 20 out of the 96 movements in each block, the hold period was extended to test the hold perturbation (4 trials for each of the 5 target locations, each one of the 4 trials testing one perturbation direction as shown in Figure 7C).
(11) Line 191. Lines 188-190. It would be useful to see a sample of several of these force traces over time (0-5s) that were used to make the average for a position. That would give insight into the stability of the forces of a participant for one of the postures. These traces could be shown in Figure 2.
Response 2.A.11. Thank you for your suggestion. We have added these panels to Figure 1, (as Figure 2 was already large). Each panel illustrates the three measurements taken at similar positions (closest to midline, distal from the body) and the same condition (paretic arm, with arm support given) for one participant (same participants as in Figure 2). Solid lines indicate the force on the x-axis (positive values indicate forces towards the left), whereas dashed lines indicate the force on the y-axis (positive values indicate forces towards the body). The shaded area indicates the part averaged in order to estimate the resting bias, illustrating how resting biases were relatively stable by the 2s mark. Note that these examples include one trial (blue traces in the third panel) which was rejected following visual inspection as described in Materials and Methods – Data Exclusion Criteria (“trials where forces appeared unstable and/or there was movement during the robot hold period”). We find this helpful as this illustrates (and motivates) one component of our methodology.
(12) Line 196. Figure 1D (not 1E).
Response 2.A.12. Thank you for catching this error, which we have now corrected.
(13) Line 215: The authors mentioned similar results. Were there any different results that impacted interpretation? Some evidence of this, similar to and in addition to Supplementary 1, would be helpful.
Response 2.A.13. We repeated our analyses without these exclusion criteria, with no impact to the interpretation. We now include versions of the main outcome panels from Figures 5, 6, and 7 in the supplementary materials calculated without this outlier exclusion (Figures S5-E, S6-E, and S7-E, respectively).
(14) Line 231: Perhaps better to explicitly state the furthest three positions are being across as the distal targets for the ANOVA.
Response 2.A.14. Thank you for your suggestion. We now explicitly clarify this in line 276:
“distal targets [furthest three positions] vs. proximal targets [closest two positions]”
(15) Figure 3B, lines 265. Clearly, these are different, but the authors should report statistics.
Response 2.A.15. We now report these numbers (lines 339-346 of the revised manuscript, which also include statistics related to bias direction as described in 2.A.17 below).
(16) Figure 2 should have a heat map scale.
Response 2.A.16. We have now added this (also Response 1.A.7), including an explanation of what the heat map represents in the caption.
(17) Figure 3C: It would be useful to quantify and plot the direction of the resting force bias vector.
Response 2.A.17. Thank you for your suggestion. We have expanded Figure 3 to include the average direction of the resting force bias vector (note the readjustment of colors following Reviewer 1’s comment: striped bars indicate No Support data, and full bars indicate Support data, with the colors being the same). The direction of the force bias vector, however, may not be very informative in cases where the magnitude is small (and the signal-to-noise ratio is small), whereas averaging the direction of the force bias vector across different positions for one participant may average out systematic variations in this direction across different locations. Nevertheless, the average direction appears generally towards the body (around -90°, or 6 o’clock) even in the non-paretic and control data (though the noise – as suggested by the size of the errorbars – is much higher in the latter cases, especially when the arm is supported). This is a (weak) suggestion that these resting biases may be present, though much subdued, in the nonparetic limb and healthy individuals; further work will be needed to elucidate this.
(18) Line 428. It is not significantly longer compared to controls. Can the authors slightly revise this sentence?
Response 2.A.18. We have revised this sentence (lines 529-532):
Patients showed impaired capacity to resist and recover from this perturbation (the abrupt release of the imposed force). The time to stabilization for the paretic side (0.94±0.05s) was longer compared to the non-paretic side (0.79±0.03s, p = 0.024) and controls (0.78±0.06s, though this was statistically marginal, p = 0.061) as shown in Figure 7E, left.
(19) Line 541. It is unclear how these data support the idea of three distinct controllers. Can the authors please clarify?
Response 2.A.19. Here, we compared our findings to previous ideas about distinct controllers, and discuss a potential fusion of these ideas with ours. Specifically, we find that holding is distinct from both initial reaching and coming to a stop. Previous work argues that initial reaching and coming to a stop are themselves distinct (Ghez et al., 2007; Jayasinghe et al., 2022). Combining these two sets of arguments, we arrive at the possibility of three distinct controllers.
(20) It would be useful if the authors provided a definition of synergy, as well as distinguishing between muscle and movement synergies.
Response 2.A.20. We now provide this in lines 591-594:
Here, “synergies” refer to abnormal co-activation patterns across joints that manifest as the patient tries to move – for example, the elbow involuntarily flexing as the patient tries to abduct their shoulder (Twitchell, 1951; Brunnstrom, 1966).
(21) Line 592-593. The wording of this sentence could be improved.
Response 2.A.21. We have switched this sentence to active voice for more clarity:
Thus, while full weight support reduces both resting flexor biases and movement-related flexor synergies, this reduction seems more complete for synergies rather than resting biases.
(22) Figure 9. In the left column, it should read normal synergies and normal resting posture.
Response 2.A.22. We intentionally used the same terminology, as the idea behind our conceptual model is that these patterns, which manifest as well-recognized abnormal synergies and abnormal resting postures in stroke, may be present in the healthy motor system as well, but kept in check by CST moderating the RST. At the same time, we recognize that, by definition, synergies and posture in controls are the “normal” reference point against which “abnormal” synergies and posture are defined after stroke. To clarify this issue, we thus decided to forgo the use of the terms “abnormal” in the figure, and instead refer to “synergistic movement ” and “synergistic resting posture”.
(23) Figure 9. With stroke, is RST upregulated, a decreased influence of CST, or both? All seem plausible.
Response 2.A.23a. We believe both can be happening. From previous work (e.g. McPherson et al., 2018) it seems safe to say that RST upregulation is the case, whereas one would also expect a decreased CST influence due to its damage due to the stroke. The relative weight of these influences would be interesting to elucidate in future work.
I have not read the paper, but did McPherson et al., 2018 test these different hypotheses?
Response 2.A.23b. The main point of McPherson et al., 2018 is that increased synergy expression is due to increased RST involvement, rather than reduced CST influence. However, McPherson et al. do not show separate increases/reductions in RST/CST activity; they show that contralesional activity relative to ipsilesional activity is increased (using a laterality index). While it does seem that RST is upregulated in this case, this does not exclude the possibility that CST influence is reduced as well.
We also noticed that the citation itself, while mentioned in the text, was missing from the bibliography. This is now fixed.
For Figure 9, McPherson is cited as they provide evidence for the idea that RST involvement increases when arm support is decreased. This evidence is both direct (e.g. in their Figure 3 where they show that “Stroke participants exhibited increased activity in the contralesional (R) hemisphere as SABD loading increased” [i.e. arm support was reduced]) and indirect: they connect synergies to RST involvement, and also show increased synergies with reduced arm support (also shown multiple times previously). Both these arguments suggest that arm support reduces RST involvement. We have clarified the relevant sentence:
The interesting implication of this conceptual model is that synergies are in fact postural abnormalities that spill over into active movement when the CST can no longer modulate the increased RST activation that occurs when weight support is removed. Supporting this idea, McPherson et al. found increased ipsilateral activity (which primarily represents activation via the descending RST (Zaaimi et al., 2012)) when the paretic arm had reduced support compared to full support (McPherson et al., 2018).
Reviewer #3 (Recommendations For The Authors):
For Experiment 2, it is not immediately clear how the within-subject values are being pooled and compared across the different conditions. For instance, in the static perturbation trials, there are four blocks with 20 perturbation trials per block per arm (80 total per arm) with each location and direction once per block. For each participant, the comparison is between the location/direction that was most opposed (although this doesn't look accurately represented in Fig 7F). Therefore, the within-subject comparison is 4 trials per participant? Were these values averaged or pooled? It is a little odd that the SD for all the within-subjects trials are identical or nearly identical across conditions especially when looking at the example patient data in 7B and 7F.
Response 3.A.1. For static perturbation trials, the within-subject comparison involves 8 trials per participant: 4 trials corresponding to the perturbation direction/position combination with resting bias most opposed to the perturbation, and 4 trials corresponding to the perturbation direction/position combination with resting bias most aligned with the perturbation. These values were averaged for each individual. We have expanded our methods to make this part of our data analysis clear (lines 284-296) for all types of comparisons (unperturbed movement, pulse perturbation, static perturbations – now referred to as “release perturbation”).
The across-subject SDs for the average resting forces for each one of these two conditions, shown in Figure 7F are indeed identical. This is due to how these two instances (most aligned vs. most resistive) were selected: because the perturbation directions come in pairs that exactly oppose each other (Figure 7B), if one were to select the position with the most opposing resting bias, that would mean that the combination with same position and the oppositely-directed perturbation would be the one with the most assistive resting bias. Hence the resting biases selected for the most opposing/assistive instances would be equal in magnitude and opposite to each other for each participant, as illustrated in Figure 7F, whereby the most-opposed bias for each individual is exactly opposite to the corresponding most-aligned bias for the same individual. We have added a brief commentary about this on the caption (lines 551-554), reproduced below:
Note how the most-opposed resting bias for each patient is equal and opposite to the their mostaligned resting bias. This is because the same resting bias, when projected along the direction of two oppositely-directed perturbations (illustrated in C), it would oppose one with the same magnitude it would align with the other.
Importantly, following suggestions by Reviewer 2 (see point 2.A.1), we now provide supplementary analyses that use the entirety of the relevant data, rather than the most extreme instances, which provide evidence supporting our main findings (Figures S5-2, S6-2, and S7-2).
The printed colors in Figure 3 are very muddled and hard to read/interpret, especially in panel A.
Response 3.A.2. Thank you for pointing out this issue, also raised by Reviewer 1. We have adjusted the colors to be more distinct from each other and look clear both in print and on-screen, making use of dashed lines and stripes rather than different shades.
I think it would improve readability and interpretation if Figure 8 and the results related to FM-UE were contained within the description of results for Experiment 1.
Response 3.A.3. Thank you for this suggestion. This is actually a debate we had among ourselves earlier, and we can see merits to either ordering. It is very arguable that moving Figure 8 and the FMUE results within the rest of Experiment 1 may improve readability somewhat. However, we believe that presenting these results at the end better serves to illustrate the apparent paradox between the lack of direct connection between resting biases and active movement on one hand, and the relationship between resting biases and abnormal synergies on the other. We believe that this better sets the stage to present our conceptual model, which explains this paradox based on the role arm support plays in modulating the expression of both resting biases and abnormal synergies.
Additional changes/corrections not outlined above
Figure 1D displayed a right arm, but showed a target array (red dots) for a left arm paradigm. We now flip the target array shown for consistency.
We corrected Figure 6C, which accidentally used an earlier definition of settling time which was based on lateral stabilization throughout the entire movement, rather focus on the period immediately following the pulse. The intended definition of settling time (as we had described in the Methods, lines 204-206 of original submission) focuses on lateral corrections specific to the pulse (rather than corrections when the participant approaches the endpoint) and better matches the one for settling time for the release (static) perturbation trials. Note that this change did not affect the (lack of) relationship between settling time and resting force bias, both across individuals (correlation plots now in Figure S6-1) and within individuals (now shown in the right part of panel 6D). Also in panel C, an error in the scaling for the maximum lateral deviation in the pulse direction (right side of the panel) is also now corrected.
In addition, we made minor edits throughout the text to improve readability.
References
Albert ST, Hadjiosif AM, Jang J, Zimnik AJ, Soteropoulos DS, Baker SN, Churchland MM, Krakauer JW, Shadmehr R (2020) Postural control of arm and fingers through integration of movement commands. Elife 9:e52507.
Avni I, Arac A, Binyamin-Netser R, Kramer S, Krakauer JW, Shmuelof L (2024) The Kinematics of 3D Arm Movements in Sub-Acute Stroke: Impaired Inter-Joint Coordination is Attributable to Both Weakness and Flexor Synergy Intrusion. Neurorehabil Neural Repair 38:646–658.
Bourbonnais D, VANDEN NOVEN S, Carey KM, Rymer WZ (1989) Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain 112:85–102.
Brunnstrom S (1966) Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 46:357–375.
Cortes JC, Goldsmith J, Harran MD, Xu J, Kim N, Schambra HM, Luft AR, Celnik P, Krakauer JW,
Kitago T (2017) A Short and Distinct Time Window for Recovery of Arm Motor Control Early After Stroke Revealed With a Global Measure of Trajectory Kinematics. Neurorehabil Neural Repair 31:552–560.
Duque J, Thonnard J, Vandermeeren Y, Sébire G, Cosnard G, Olivier E (2003) Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 126:732–747.
Fine MS, Thoroughman KA (2006) Motor Adaptation to Single Force Pulses: Sensitive to Direction but Insensitive to Within-Movement Pulse Placement and Magnitude. J Neurophysiol 96:710–720.
Ghez C, Scheidt R, Heijink H (2007) Different Learned Coordinate Frames for Planning Trajectories and Final Positions in Reaching. J Neurophysiol 98:3614–3626.
Hadjiosif AM, Branscheidt M, Anaya MA, Runnalls KD, Keller J, Bastian AJ, Celnik PA, Krakauer JW (2022) Dissociation between abnormal motor synergies and impaired reaching dexterity after stroke. J Neurophysiol 127:856–868.
Jayasinghe SA, Scheidt RA, Sainburg RL (2022) Neural Control of Stopping and Stabilizing the Arm. Front Integr Neurosci 16.
Kanade-Mehta P, Bengtson M, Stoeckmann T, McGuire J, Ghez C, Scheidt RA (2023) Spatial mapping of posture-dependent resistance to passive displacement of the hypertonic arm post-stroke. J NeuroEngineering Rehabil 20:163.
Lawrence DG, Kuypers HG (1968) The functional organization of the motor system in the monkey: II. The effects of lesions of the descending brain-stem pathways. Brain 91:15–36.
Levin MF (1996) Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain 119:281–293.
Lowrey CR, Bourke TC, Bagg SD, Dukelow SP, Scott SH (2019) A postural unloading task to assess fast corrective responses in the upper limb following stroke. J NeuroEngineering Rehabil 16:1–17.
McPherson JG, Chen A, Ellis MD, Yao J, Heckman C, Dewald JP (2018) Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol 596:1211–1225.
McPherson LM, Dewald JP (2022) Abnormal synergies and associated reactions post-hemiparetic stroke reflect muscle activation patterns of brainstem motor pathways. Front Neurol 13:934670.
Porter R, Lemon R (1995) Corticospinal function and voluntary movement. Oxford University Press.
Smith MA, Brandt J, Shadmehr R (2000) Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 403:544.
Smith MA, Shadmehr R (2005) Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol 93:2809–2821.
Tower SS (1940) Pyramidal lesion in the monkey. Brain 63:36–90.
Twitchell TE (1951) The restoration of motor function following hemiplegia in man. Brain 74:443–480.
Wilkins KB, Yao J, Owen M, Karbasforoushan H, Carmona C, Dewald JP (2020) Limited capacity for ipsilateral secondary motor areas to support hand function post-stroke. J Physiol 598:2153– 2167.
Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN (2012) Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135:2277–2289.