Author response:
The following is the authors’ response to the original reviews.
We thank the reviewers for their constructive comments on our manuscript and their appreciation of the results. We provide point-by-point responses bellow. For your convenience we highlight here the main changes to the manuscript.
· More descriptive terminology for the contextual cues (Ctx.A / Ctx.noA is now referred to as LIGHT / DARK).
· Schematic of experiment timeline highlighting the exclusion of non-discriminators following the initial acquisition period. This explains the absence of baseline sex differences post acquisition and clears up some misconceptions about lack of replicability.
· New data (time in port preCS) showing that a prior reward does not cause continued presence in port.
· Several text edits to address all the points raised by the reviewers.
We hope that the editors and reviewers will be satisfied with this revised version and find the strength of the evidence more convincing.
Reviewer #1 (Recommendations For The Authors):
In relation to weaknesses points 1-4 in the public review:
(1) With regards to the claim (page 4 of pdf), I think I can see what the authors are getting at when they claim "Only Ctx-dep.01 engages context-gated reward predictions", because the same reward is available in each context, and the animal must use contextual information to determine which cue will be rewarded. In other words, it has a discriminative purpose. In Ctx-dep.O1/O2, however, although the context doesn't serve a discriminative purpose in the sense that one cue will always earn a unique outcome, regardless of context, the fact that these cues are differentially rewarded in the different context means that animals may well form context-gated cue-outcome associations (e.g. CtxA-(CS1-O1), CtxnoA-(CS2-O2)). Moreover, the context is informative in this group in telling the animal which cue will be rewarded, even prior to outcome delivery, such that I don't think contextual information will fade to the background of the association and attention be lost to it in the way, say Mackintosh (1975) might predict. Therefore, I don't think this statement is correct.
I suggest that the authors refine the statement to be more accurate.
We agree with the reviewer —the context is absolutely relevant for rats trained in the Ctx-dep. O1/O2 task. We have edited the text in several places to make this clear. The question is how (by what mechanism) does the context participate in the control of behavior in this group. The reviewer correctly points out that, just like rats trained in the Ctx-dep. O1 task, rats trained in the Ctx-dep. O1/O2 might have formed context-gated cue-outcome associations. We now clearly acknowledge that in the text.
However, because in this group the two outcomes are always encountered in different contexts, we argue that these rats could also have formed a direct association between the two contexts and the two outcomes. In other words, each context might directly evoke the expectation of a distinct reward outcome (prepare to drink, or prepare to eat). On a given trial, if the cue and context both tend to activate the same outcome representation, the converging cue+context excitation can add up. This would produce a context-sensitive response, but not via hierarchical modulation process (unlike Ctx-dep O1). Arguably, this last associative mechanism is much simpler and might explain why almost all rats in Ctx-dep. O1/O2 group learned the discrimination and at a much faster rate.
Therefore, while rats trained in Ctx-dep O1/O2 might engage a combination of associative processes to achieve context-sensitive behavior (including hierarchical associations), only rats in the Ctx-dep O1 critically and unambiguously rely on hierarchical associations to achieve context-sensitive behavior.
(2) I think the results shown in Figure 1 are very interesting, and well supported by the statistics. It's so nice to see a significant interaction, as so many papers try to report these types of effects without it. However, I do wonder how specific the results are to contextual modulation. That is, should a discriminative discrete cue be used instead of each context (e.g. CS1 indicates CS2 earns O1, CS3 indicates CS4 earns O1), would female rats still be as slow to learn the discrimination?
I am just curious as to whether the authors have thoughts on this.
We have not tested this and are not aware of a paper that examined this question specifically.
However, we would like to point out that in the suggested design (CS1→[CS2→O1]; CS3→[CS4→O1]) the discriminative cues (CS1 and CS3) would almost certainly also acquire substantial reward-predictive value, either because of their direct association with the reward, or via second-order conditioning. This would complicate the interpretation of the results in terms of hierarchical associations. Incorporating non-rewarded presentation of CS1 and CS3 alone (i.e. extinguishing those cues, as is sometimes done in occasion setting experiments) would be one way to reduce the reward expectation evoked by those cues, but this approach has some limitations. Indeed, as mentioned by Rescorla (2006) “During extinction, the net associative strength of a stimulus declines to the level of [a response] threshold, but further decrement stops at that point”. So while extinguished CS1 and CS3 might no longer evoke overt behavioral responses, these cues could retain nonnegligible subthreshold excitatory connection with the US. Individually, these cues might fail to evoke responding but could nonetheless increase responding during the CS1→CS2 trials (or CS3→CS4 trials), via simple summation. (Rescorla, 2006: “the compound of two [extinguished] stimuli has a strength that exceeds the threshold and so evokes responding”).
This type of consideration is precisely why we opted for the behavioral task used in the study. In Ctx-dep. O1, the discriminative stimuli exert opposite effects on the two target cues, which rules out summation effects as a mechanism for context-sensitive behavior.
(3) Pages 8-9 of pdf, where the biological basis or the delayed acquisition of contextual control in females is considered, I find this to be written from a place of assuming that what is observed in the males is the default behaviour. That is, although the estrous cycle and its effects on synaptic plasticity/physiology may well account for the results, is there not a similar argument to be made for androgens in males? Perhaps the androgens also somehow alter synaptic plasticity/physiology, leading to their faster speed, reduced performance stability, and increased susceptibility to stress.
I would like the argument that female behaviour might be the default, and male behaviour the deviation to be considered in the discussion in addition to those already stated.
We regret if we gave the impression that male behavior was the default. The paper is intended to report sex differences but we don’t view either sex as the default. To correct this impression, we have added a few sentences in the discussion to highlight male-hormonal factors as well as non-gonadal genetic factors that might have contributed to the observed sex differences.
(4) In addition, the OFC - which is the brain region found to have differential expression of c-fos in males and females in Figure 5 - is not explicitly discussed with regard to the biological mechanisms of differences, which seems odd.
I suggest OFC be discussed with regard to biological mechanisms of differences.
We added a few sentences in the discussion to i) highlight the parallel between our study and human fMRI studies showing superior OFC activation in females during the regulation of emotional responses, ii) Suggest a potential relationship between the reported sex differences (speed of acquisition, robustness of performance, and OFC activation in context-gated reward prediction), iii) acknowledge our ignorance of the root causes of these sex differences.
We wish we could offer a better answer. We have attempted to offer possible proximal explanations for the observed sex differences, but ultimately our work did not address the root causes of these behavioral and neural sex differences. Therefore we feel that further attempts to explain these differences would be too speculative.
(5) I did wonder if the authors were aware that in the Rescorla-Wagner model, contextual stimuli are thought to summate with discrete cues to enter into the association with the outcome (i.e., the error term is between lambda and sigmaV, with sigmaV the 'summation' of all stimuli present on a trial, including contextual stimuli). Typically, this is not considered much, because the cue itself is so salient and more consistently paired with reward (whereas the ever-present context is often paired with no reward), but nevertheless, it is a part of the association. I'm not sure it's wrong to say that the background circumstances under which events occur are thought to play little role (as in the second sentence of the introduction), but I was wondering if the authors were aware of this fact when they wrote that.
This sentence in the introduction was meant to introduce the distinction between eliciting stimuli and modulating contexts. Admittedly, this paints a naive picture, which we now acknowledge (we hope that the rest of the paper provides more nuance). As pointed out by this reviewer, the context is also a stimulus, and, just like any other stimulus, it is eligible for direct association with an outcome. The possibility for direct context→outcome association is precisely the rational for the Ctx-dep O1/O2 group.
(6) Context-noA - Seems a little confusing for a name, why not just call it context B? NoA appears to imply that nothing happens in A or no outcome is available, whereas this is not always the case.
We debated which terminology to use. We felt that “Context A vs. Context B” should perhaps be reserved to situations where the global context changes (e.g. two different conditioning boxes with different odors, floor texture etc., with proper counterbalancing procedures). We felt that “Context A vs noA” might be more appropriate here, as we are manipulating the local context by introducing (or removing) one single stimulus (the houselight). In this revised version we followed this reviewer’s advice and adopted a more descriptive, and hopefully less confusing, terminology: "Light vs Dark”.
(7) Why is it that in the text the Ctx-dep O1/O2 is explained before simple and no discrimination, but in the Figure Ctx-dep O1/O2 is shown last? These should be consistent.
Thanks for pointing that out. We have switched the order of task description to be consistent with the figures.
(8) Page 6 (of pdf) - could the authors elaborate a little on why or how (or both) the delivery of reward can interfere with the expression of context-dependent discrimination? Do they just mean the performance of discrimination (e.g., animals will sit at the food port longer if there is food there because they are sitting there and eating it, which does not necessarily reflect the expectation of food based on cue presentations?), in which case it is not the discrimination itself that is being interfered with, just the measure of it. Perhaps the authors could elaborate by just inserting a sentence.
We have added a few sentences to discuss this effect.
The first clarification that we can make is that the reduced discrimination performance following reward is not simply due to animals’ continued presence in the reward port. We have added the time pre-cue to Fig. 3 B-F. This measure is not affected by previous reward history, showing that rats are leaving the port between trials.
So what is driving this effect? At this stage, we are agnostic about the mechanism(s) for this effect. Kuchibhotla et al. (2019) —who first reported a similar effect— proposed a model in which recent rewards modify the threshold for behavioral responses (i.e. performance). In this model, a cue might evoke a weak reward prediction but evoke a strong behavioral response if presented after a reward. Additionally, we believe that learning factors might also contribute to the effect reported here. Indeed, the behavioral response on a given trial likely reflects the balance of hierarchical (context-dependent) associations vs. direct associations (Bradfield and Balleine, 2013). Naturally, this balance is dynamic and influenced by trial history. For instance, a Light:X+ trial might increase the value of cue X and promote responding during the following Dark:X- trial. The same logic could be applied to the influence of the context (e.g., Light:X+ trial might promote responding to a subsequent Light:Y- trial). We are currently working on a computational model that captures the dynamic interplay between hierarchical associations and direct associations. We hope that this model will provide some insight into the learning/performance mechanism for the effects reported here. However this computational work is still in the early stages and beyond the scope of the present study.
(9) The lack of effect in the Ctx-dep O1/O2 groups in Figure 4 could be due to a lack of power - the group sizes are a lot smaller for this group than for Ctx-dep O1 where an interaction was detected. I think this should be at least addressed in the discussion (i.e., that this lack of effect is possibly due to less power here, as the effects are in the same direction).
Good point. We now acknowledge this limitation in the text.
Reviewer #2 (Recommendations For The Authors):
(1) Please comment on the failure to replicate the sex differences across experiments. Perhaps this is due to some change in the training procedure that is briefly mentioned in the methods (a reduction in the number of rewarded trials) but it is unclear.
The reviewer correctly observed that Fig. 3-5 do not show sex differences in baseline condition. This is not because of a replication failure, but because non-discriminating subjects were excluded from the experiment at the end of the acquisition period (after 72 training sessions). We now clarify this in the Method and Results section. We also added a schematic of the experiment timeline that highlights the exclusion of non-discriminators at the end of the acquisition period (Fig 1).
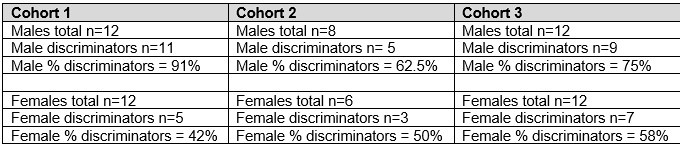
On the topic of replicability, the data for Ctx-dep O1 was collected over 3 cohorts (over the course of 2 years) and the sex difference pattern was consistent. For instance, the proportion of discriminators vs. non-discriminators for males and females trained in Ctx-dep O1, showed similar patterns across cohorts (see below).
Author response table 1.
(2) The design of this experiment makes it possible to analyse whether there is a differential outcome effect (DOE). The DOE would indeed predict better discrimination in group cxt-dep O1/O2 versus cxt-dep O1, which seems to be exactly what the authors observe although between-group statistics are not reported. Inspection of Figure 1 suggests that there may be a DOE in females but not in males. I wonder if the authors might consider reanalysing the data to check this.
Indeed, there is clearly a differential outcome effect. We now point out this DOE in relation to the latency to achieve discrimination criterion (Fig. 2 C-D). Rats in the Ctx-dep. O1/O2 group acquired discrimination (reached criterion) much faster than rats in in the Ctx-dep. O1 group.
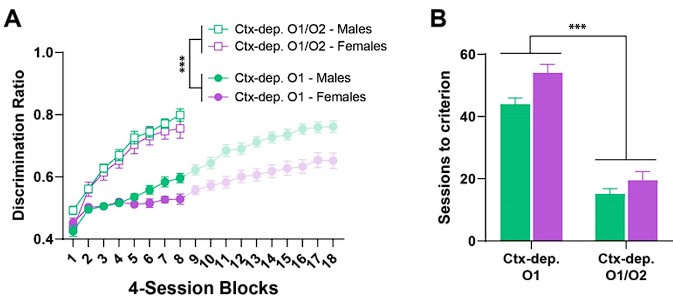
Following the reviewer’s suggestion, we provide here the results of targeted ANOVAs (focusing exclusively on Ctx-dep. O1 and Ctx-dep. O1/O2) to investigate a potential sex-dependent effect of DOE (i.e. Sex x Task interactions), see figure below. A three-way ANOVA (Sex x Task x Session) conducted on the discrimination index reveal a main effect of Task (F1, 86 = 173.560, P < 0.001), Session (F2.678, 230.329 = 140.479, P<0.001) and a marginal effect of Sex (F1,86 = 3.929, P = 0.051), but critically no Task x Sex or Task x Sex x Session interaction (P ≥ 0.504). A two-way ANOVA (Sex x Task) conducted on the sessions to criterion revealed a main effect of both factors (Sex F1, 63 = 9.52, P = 0.003; Task F1, 62 = 184.143, P < 0.001) but critically, no Sex x Task interaction (P = 0.233). These results indicate that the use of two different outcomes clearly facilitated the acquisition of context-dependent discrimination (DOE effect), but this effect benefited both sexes equally. We thank the reviewer for recommending this analysis.
Author response image 1.
Differential outcome effect (DOE) affects males and females equally. A. Discrimination ratio over the acquisition period. B. trials to criterion. Compared to animals trained with a single outcome (Ctx-dep. O1), the introducing dissociable outcomes for the two type of rewarded trials (Ctx-dep. O1/O2) profoundly facilitated the acquisition of discriminated behavior. This effect benefited both sexes equally.
(3) Some minor points for clarification that the authors may also wish to address:
- Figure 3: is data presented from sessions 71-80 only or for all sessions? I didn't fully follow the explanation offered in the results section.
That’s right. The data presented in Fig. 3 considers only sessions 71-80, in discriminator rats —when performance is globally stable. We have edited the text to make this clearer. These 10 sessions represent a total of 800 trials (=10 session * 80 trials). The first trial of a session what not included in the analysis since it was not preceded by any trial. For the remaining 790 trials (10 session x 79 trials), we examined how the outcome of the past trial (reward or nonrewarded) influenced responding on the next trial. This large sample size (790 trials / rat) was required to ensure that enough data was collected for each possible trial history scenario.
- The authors argue that females are protected from the disrupting effect of stress. It might be useful if the authors offer further explanation as to what they mean by "protected".
By “protected”, we simply mean “less sensitive”. We have reworded this sentence in that way. We do not claim to have an understanding of the precise mechanism for this sex dependent effect (although our data point to a possible role of the OFC).
- The authors state that "delivery of reward, while critical for learning, can also interfere with the expression of context-dependent discrimination". This statement should be explained in further detail. For instance, why should reward delivery specifically impair context-dependent discrimination but not other forms of discrimination?
We have reworded this sentence to be more inclusive. Indeed, delivery of reward also interferes with other forms of discrimination, particularly when discrimination performance is not yet optimal. We have also added a paragraph to discuss the possible mechanisms by which reward might interfere with discrimination performance in our task.
Reviewer #3 (Recommendations For The Authors):
I do not suggest additional experiments, but I do hope you continue the behavioral work to characterize what is being learned in the task. I think the approach is promising. I would suggest reporting the % time in port and port entries for the entire CS. There is no justification for only analyzing the response in the last 5s.
We thank the reviewer for the encouragement.
We opted to focus on the time in port for two main reasons:
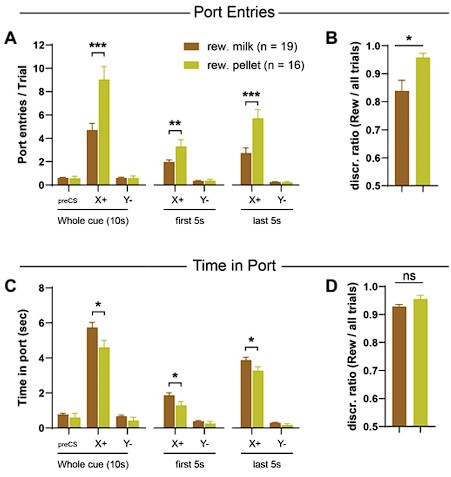
(1) This measure is relatively consistent across the two different reward outcomes (unlike the rate of port entries). Indeed, consistent with prior studies (Delamater et al., 2017), we observed that the type of reward (solid or liquid) influences the topography of the anticipatory magazine-directed behavior. Specifically, cues paired with pellets elicited significantly more port entries than cues paired with chocolate milk. The opposite pattern was observed for time in port --cues paired with chocolate milk elicited more sustained time in port compared to cues paired with pellets (see figure below). While these measures (port entries and time in port) show opposite bias for the two possible outcomes, the size of this bias is much smaller for the time in port (Cohen’s d effect size: port entries: 1.41; time in port: 0.62). As a result, the discrimination ratio calculated from Time in port is consistent across the two outcomes (P = 0.078; effect size: 0.07), which is not the case for the discrimination ratio calculated from port entries (P = 0.007; effect size 0.32 see figure below).
(2) Unlike the rate of port entries, the time in port shows monotonic increase during training in these tasks. Indeed, we observed here and in past work (Keiflin et al., 2019), that the rate of port entries initially increases with training, but then slightly decreases; particularly for cues paired with liquid reward. In contrast, the time in port continues to increase, or remains high, with extended training. This is easy to understand if we consider the extreme case of a hypothetical rat that might enter the port once upon cue presentation and maintain continued presence in port for the whole cue duration. This rat would have a relatively low rate of port entry (a single port entry per trial) but a high time in port.
This is not to say that the rate of port entries is not a valid measure overall (we have used, and continue to use, this metric in other preparations). However, for the reasons explained above, we believe that the time in port is a better metric for reward anticipation in this specific study.
Moreover, we chose to focus our analysis on the last 5s of the cue because that’s when anticipatory food cup behavior is more reliably observed (in our preparation >2/3 of the total time in port in occurs during the last 5s of the cue) and less contaminated by orienting behaviors (Holland, 1977, 1980, 2000). For these reasons, analysis of the last portion of the cue is relatively common in Pavlovian anticipatory approach preparations (El-Amamy and Holland, 2007; Olshavsky et al., 2013; Esber et al., 2015; Holland, 2016a, 2016b; Schiffino and Holland, 2016; Gardner et al., 2017; Sharpe et al., 2021; Maes et al., 2020; Sharpe et al., 2020; Siemian et al., 2021; Kang et al., 2021). Reporting time in port during the same cue epoch facilitates comparisons between these studies.
We have edited the text in the Method section to provide a brief justification for focusing our analyses on this cue epoch.
Author response image 2.
Outcome identity influences the topography of the conditioned response. A-C: Conditioned responding expressed as the number of port entries per trial (A) or time in port per trials (C) for rats trained in the simple discrimination task with a chocolate milk reward (n= 19) or a sucrose pellet (n = 16). Data show the average of the last three 3 sessions. Compared to chocolate milk, pellets tend to produce more port entries. Conversely, chocolate milk tend to produce more time in port. However the magnitude of this bias is smaller for the Time in port. C-D: discrimination ratio calculate from the number of port entries (C) or the time in port (D); the latter is not affected by the outcome identity. *P<0.05; **P<0.01; ***P<0.001 T tests.
The inconsistent use of terms is distracting throughout the paper. Is it discriminated or context-gated? Please provide a definition of your terms and then use them consistently. Is it a discriminative stimulus, a context, or an occasion setter? These all imply slightly different things and it would help the reader if you just used one term throughout the paper.
Thanks for pointing that out. We have added a definition for “context-gated” and edited the text to keep the terminology consistent when appropriate. The words “discrimination”/”discriminated” still appear in the manuscript but without implying a mechanism (all tasks are variations of Pavlovian discrimination; the rats discriminating between rewarded and non-rewarded trials).
As mentioned by this reviewer, the terms “context” and “occasion setter” are not synonymous. Therefore these terms still appear in the manuscript to refer to different concepts (e.g. in our task the visual stimulus is a context for all rats; this context acts as an occasion setter only for some rats).
Minor:
Intro, 2nd PP: "autism". This is abbreviated in the abstract but spelled out here. I suggest not abbreviating in the abstract and introducing abbreviations here, as you do with PTSD.
Fixed as suggested
Have deficits in contextual modulation been distinguished from potential deficits in binary associative learning in autism, PTSD, and substance use disorders? This is implied, but there are no citations provided.
We provide a list of references showing deficits in contextual modulation in these disorders.
This does not mean that these disorders are reducible to deficits in contextual modulation and it does not exclude other forms of deficits in those disorders --including alterations in certain aspects of binary associative learning.
"In positive occasion-setting, animals learn that a target cue (X) results in a reward outcome (+) only when that cue is accompanied by a contextual feature (A); the same cue presented in absence of this contextual feature remains without consequence (A:X+ / X-)." - there are words missing in this sentence.
We apologize but we fail identify the missing word(s). Perhaps the reviewer could be more specific and we will be happy to edit the sentence as needed.
What is a contextual feature, is this redundant or can you provide a specific definition?
We use the terminology “feature” and “target” as these are the standard terms in the description of occasion setting preparations (one stimulus, “the feature”, sets the occasion for responding –or not responding- to the “target” cue). By contextual feature, we meant that in this specific example the context was the feature. We have clarified this in the text. We believe that these terms are not redundant. Indeed, the context is not always a feature, and a feature is not necessarily a context (phasic cues can serve as “features”).
Can you provide some background on studies of sex differences in simple associative learning? You imply these have been much more thoroughly studied than conditional discriminations.
We added a few references as suggested.
What is the rationale for studying stress?
Stressful life events exacerbate several mental illnesses, potentially by impacting cognitive functions.
Although the (sex-dependent) effects of stress on some cognitive function are well established (e.g. working memory, selective attention, spatial navigation), the effect of stress on contextual modulation (a core dysfunction in certain mental illnesses) --and the possible sex-differences in this effect-- had not been formally tested. We added a few sentences in the results section (at the beginning of the stress section) to remind the reminder of why we tested the effect of stress in this task.
Method/Results:
Cues are not counterbalanced; the feature is visual and targets are auditory - this should be noted as a limitation in the discussion section.
We now acknowledge this limitation in the discussion. Moreover we believe that the new terminology for the context —Light vs Dark— (instead of A vs. noA in the original version) makes it abundantly clear that the “context” is this study was always visual.
Summation is invoked to describe the discrimination with different outcomes, how is summation happening? This is not described. Perhaps incorporate the literature on conditional discriminations with differential outcomes (the "differential outcomes effect").
We have edited the Result + Discussion section to clarify how summation might contribute to discrimination with different outcomes. We have also added references for the DOE in this task.
The stress effect is confounded with test order; comparing stress vs. baseline.
Sorry we don’t understand this point. The “baseline” refers to the animal’s performance on the last training session before the acute stress manipulation (we have edited the text to make this clear). Animals are first trained in the task and then we examine how stress alters their performance in this learned task. We don’t see how this could induce a test order confound.
Throughout the results section, it would be helpful to have the number of animals reported for each analysis.
The number of animals for each part of the experiment is now reported in the text, as well as in the figures.
Discussion:
"For Ctx-dep. O1, context is an occasion-setter, i.e. a stimulus that hierarchically modulates the associative strength between a target cue and its outcome." This is inaccurate. Occasion setters do not change or modulate the associative strength of a target cue. They modulate whether excitation or inhibition is expressed.
We reworded the sentence as suggested: “For Ctx-dep. O1, context is an occasion-setter, i.e. a stimulus that modulates the response to a target cue”.
"Together, these results indicate that the sex differences observed here are not attributable to simple associative, motivational, working-memory, or attentional processes, but are specific to the neurocomputational operations required for the hierarchical, contextual control of behavior." It should be noted here that the difference is one of degree, a quantitative difference, but not a difference in the qualitative features of the process.
"Regardless of the precise mechanism, our results indicate that, compared to male rats, females ultimately achieved more stable contextual control over cued reward-seeking; their behavior remained context-regulated under stress or after recent rewards." Again this is a matter of degree.
We absolutely agree. All the sex-difference reported here are a matter of degree. In the framework of McCarthy et al. (2012) the reported effects are type 2 or type 3 sex differences, not type 1 sexual dimorphism. We made a few edits in the Discussion to clarify this point.
Procedure:
Please clarify the percentage of trials that were reinforced in the No Discrimination group.
From session 1-32 (acquisition period), 50% of the trials were reinforced. Following this acquisition period, only 25% of the trials were reinforced to match all the other groups. We have edited the method section to clarify this point.
Please provide the dimensions of the restraint tubes and the model number if available.
This information is now included.
References
Bradfield LA, Balleine BW (2013) Hierarchical and binary associations compete for behavioral control during instrumental biconditional discrimination. J Exp Psychol Anim Behav Process 39:2–13.
Delamater AR, Garr E, Lawrence S, Whitlow JW (2017) Elemental, configural, and occasion setting mechanisms in biconditional and patterning discriminations. Behav Processes 137:40–52.
El-Amamy H, Holland PC (2007) Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci 25:1557–1567.
Esber GR, Torres-Tristani K, Holland PC (2015) Amygdalo-striatal interaction in the enhancement of stimulus salience in associative learning. Behav Neurosci 129:87–95.
Gardner MPH, Conroy JS, Shaham MH, Styer CV, Schoenbaum G (2017) Lateral Orbitofrontal Inactivation Dissociates Devaluation-Sensitive Behavior and Economic Choice. Neuron 96:1192–1203.e4.
Holland PC (1977) Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process 3:77–104.
Holland PC (1980) CS-US interval as a determinant of the form of Pavlovian appetitive conditioned responses. J Exp Psychol Anim Behav Process 6:155–174.
Holland PC (2000) Trial and intertrial durations in appetitive conditioning in rats. Anim Learn Behav 28:121–135.
Holland PC (2016a) Enhancing second-order conditioning with lesions of the basolateral amygdala. Behav Neurosci 130:176–181.
Holland PC (2016b) Effects of amygdala lesions on overexpectation phenomena in food cup approach and autoshaping procedures. Behav Neurosci 130:357–375.
Kang M, Reverte I, Volz S, Kaufman K, Fevola S, Matarazzo A, Alhazmi FH, Marquez I, Iordanova MD, Esber GR (2021) Agency rescues competition for credit assignment among predictive cues from adverse learning conditions. Sci Rep 11:16187.
Keiflin R, Pribut HJ, Shah NB, Janak PH (2019) Ventral tegmental dopamine neurons participate in reward identity predictions. Curr Biol 29:93–103.e3.
Kuchibhotla KV, Hindmarsh Sten T, Papadoyannis ES, Elnozahy S, Fogelson KA, Kumar R, Boubenec Y, Holland PC, Ostojic S, Froemke RC (2019) Dissociating task acquisition from expression during learning reveals latent knowledge. Nat Commun 10:2151.
Maes EJP, Sharpe MJ, Usypchuk AA, Lozzi M, Chang CY, Gardner MPH, Schoenbaum G, Iordanova MD (2020) Causal evidence supporting the proposal that dopamine transients function as temporal difference prediction errors. Nat Neurosci 23:176–178.
McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012) Sex differences in the brain: the not so inconvenient truth. J Neurosci 32:2241–2247.
Olshavsky ME, Song BJ, Powell DJ, Jones CE, Monfils M-H, Lee HJ (2013) Updating appetitive memory during reconsolidation window: critical role of cue-directed behavior and amygdala central nucleus. Front Behav Neurosci 7:186.
Rescorla RA (2006) Deepened extinction from compound stimulus presentation. J Exp Psychol Anim Behav Process 32:135–144.
Schiffino FL, Holland PC (2016) Secondary visual cortex is critical to the expression of surprise-induced enhancements in cue associability in rats. Eur J Neurosci 44:1870–1877.
Sharpe MJ, Batchelor HM, Mueller LE, Gardner MPH, Schoenbaum G (2021) Past experience shapes the neural circuits recruited for future learning. Nat Neurosci 24:391–400.
Sharpe MJ, Batchelor HM, Mueller LE, Yun Chang C, Maes EJP, Niv Y, Schoenbaum G (2020) Dopamine transients do not act as model-free prediction errors during associative learning. Nat Commun 11:106.
Siemian JN, Arenivar MA, Sarsfield S, Borja CB, Russell CN, Aponte Y (2021) Lateral hypothalamic LEPR neurons drive appetitive but not consummatory behaviors. Cell Rep 36:109615.






