Author response:
The following is the authors’ response to the previous reviews.
Reviewer #3:
Comments on current version:
As mentioned in my first review, this work is significantly underpowered for the following reasons: 1) n=4 for each treatment group.; 2) no randomization of the surgical sites receiving treatments; 3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
We sincerely appreciate your thorough review and valuable feedback. We have carefully considered your comments and would like to address them as follows:
As mentioned in my first review, this work is significantly underpowered for the following reasons:
(1) n=4 for each treatment group.;
We acknowledge your concern regarding the limited sample size (n=4 per group). While we understand this may affect statistical power, our choice was influenced by ethical considerations in animal experimentation and resource constraints. Increasing the sample size would undoubtedly strengthen the statistical power of our study. However, the logistical and ethical constraints associated with using a larger number of animals in such invasive procedures were significant limiting factors. Specifically, increasing the number of medium to large experimental animals could raise ethical issues, so we used the minimum number possible. Additionally, our study design was reviewed and approved by the animal IRB, which dictated the minimum number of animals we could use. Nevertheless, we conducted power analysis to ensure that our sample size, although limited, was sufficient to detect significant differences given the high variability typically observed in biological responses. The results obtained from our n=4 samples showed consistent trends and significant differences between groups, indicating the robustness of our findings. I will include this point in the limitations section of the discussion. Thank you.
(2) no randomization of the surgical sites receiving treatments;
Thank you for pointing out this issue. We agree that randomization is essential when considering individual differences and the anatomical variations of the jawbone, such as those found in humans. However, this study is an animal experiment where other conditions were controlled, and the interventions were applied after complete bone healing following tooth extraction. Therefore, the impact of randomization of surgical sites was likely minimal, and it is challenging to determine whether it significantly influenced the experimental results. Of course, twelve female OVX beagles were randomly designated into three groups. (Methods section, line 298) However regarding your concern, we would like to present the robustness of histological results from different surgical sites as shown below. Also we will include this point in the limitations section of the discussion.
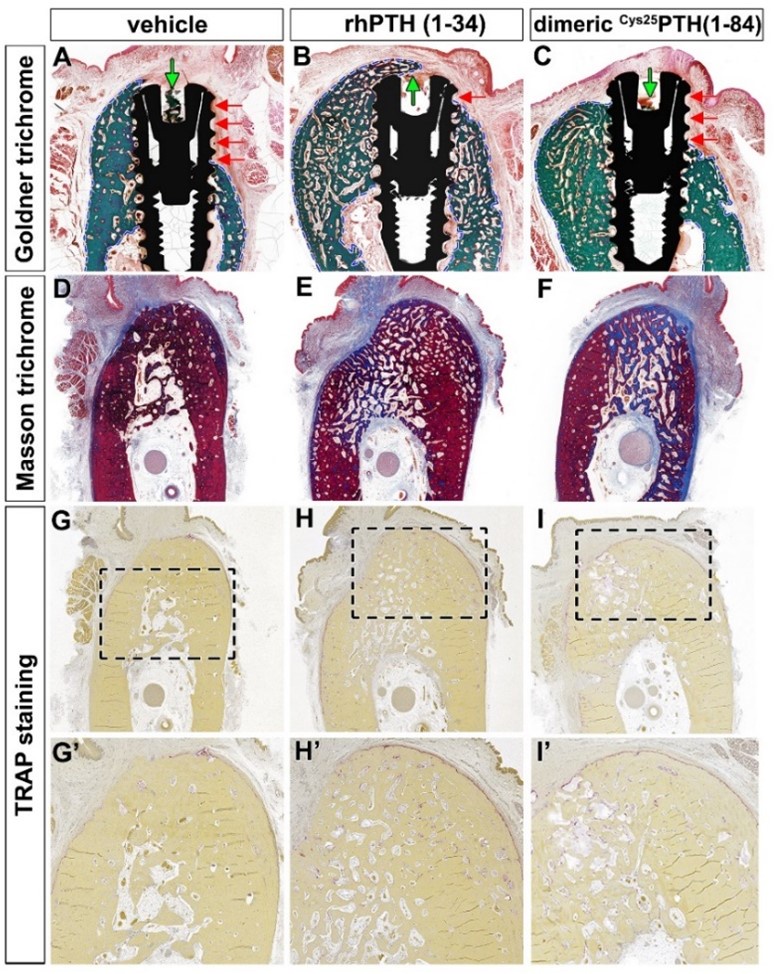
Histologic analysis of the different surgical sites showed significant differences in bone formation and osseointegration among the three treatment groups: vehicle control, rhPTH(1-34), and dimeric Cys25PTH(1-34). Goldner trichrome staining (Figure A-C) showed enhanced bone formation in both the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups compared to the vehicle control group. The rhPTH(1-34) group showed the most pronounced bone mass gain around the implant. Both treatment groups showed improved bone-to-implant contact compared to the control group, as indicated by the red arrows.
Masson trichrome staining (Figure D-F) further confirmed these results, showing an increase in bone matrix (blue staining) in the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups, with the dimeric rhPTH(1-34) group showing the most extensive and dense bone formation.
TRAP staining (Figure G-I and G'-I') was used to assess osteoclast activity. Interestingly, both the rhPTH(1-34) and dimeric Cys25PTH(1-34) groups showed an increase in TRAP-positive cells compared to the vehicle control, suggesting enhanced bone remodeling activity. The highest number of TRAP-positive cells was observed in the rhPTH(1-34) group and the highest trabecular number, indicating the most active bone remodeling.
To summarize the results, histological analyses revealed that both rhPTH(1-34) and dimeric Cys25PTH(1-34) treatments significantly enhanced osseointegration and bone formation around titanium implants in a postmenopausal osteoporosis model compared to the control. The rhPTH(1-34) group demonstrated superior outcomes, exhibiting the most substantial increase in bone volume, bone-to-implant contact, and osteoclastic activity, indicating its greater efficacy in promoting bone regeneration and implant integration in this experimental context.
Author response image 1.
Histological analysis using Goldner trichrome, Masson trichrome, and TRAP staining
(3) implants surgically inserted without precision/guided surgery. The authors have not addressed these concerns.
The primary purpose of precision guides is to prevent damage to various anatomical structures and to ensure perfect placement at the desired location. Even disregarding the potential inaccuracies of precision guides in actual clinical settings, the primary goal of this animal experiment was not to achieve perfect placement or prevent damage to anatomical structures. Instead, the objective was to histologically measure the integrity of the bone surrounding titanium fixture's platform after pharmacological intervention, ensuring it was fully seated in the alveolar bone. To this end, we secured sufficient visibility through periosteal dissection to confirm the perfect placement of the implant and adhered to the principle of maintaining sufficient mesiodistal distance between each fixture. Using such precision guides in this animal experiment, which is not an evaluation of 'implant precision guides,' could potentially introduce inaccuracies and contradict the experimental objectives. Furthermore, since this experiment was conducted on an edentulous ridge where all teeth had been extracted, achieving the same placement as in the presurgical simulation would be impossible, even with the use of precision guides. Thank you once again for your constructive feedback. We will include this point in the limitations section of the discussion.
On a minor note: not sure why the authors present a methodology to evaluate the dynamic bone formation (line 272) but do not present results (i.e. by means of histomorphometrical analyses) utilizing this methodology.
As the reviewer mentioned, we confirmed that the sentence was included in the Methods section despite the analysis not actually being performed. We sincerely apologize for this oversight and will make the necessary corrections immediately. Thank you very much for your keen observation.




