Peer review process
Revised: This Reviewed Preprint has been revised by the authors in response to the previous round of peer review; the eLife assessment and the public reviews have been updated where necessary by the editors and peer reviewers.
Read more about eLife’s peer review process.Editors
- Reviewing EditorAlyssa WilsonIcahn School of Medicine at Mount Sinai, New York, United States of America
- Senior EditorSonia SenTata Institute for Genetics and Society, Bangalore, India
Joint Public Review:
Summary
This manuscript explores the transcriptomic identities of olfactory ensheathing cells (OECs), glial cells that support life-long axonal growth in olfactory neurons, as they relate to spinal cord injury repair. The authors show that transplantation of cultured, immunopurified rodent OECs at a spinal cord injury site can promote injury-bridging axonal regrowth. They then characterize these OECs using single-cell RNA sequencing, identifying five subtypes and proposing functional roles that include regeneration, wound healing, and cell-cell communication. They identify one progenitor OEC subpopulation and also report several other functionally relevant findings, notably, that OEC marker genes contain mixtures of other glial cell type markers (such as for Schwann cells and astrocytes), and that these cultured OECs produce and secrete Reelin, a regrowth-promoting protein that has been disputed as a gene product of OECs.
Strengths
This manuscript offers an extensive, cell-level characterization of OECs, supporting their potential therapeutic value for spinal cord injury and suggesting potential underlying repair mechanisms. The authors use various approaches to validate their findings, providing interesting images that show the overlap between sprouting axons and transplanted OECs, and showing that OEC marker genes identified using single-cell RNA sequencing are present in vivo, in both olfactory bulb tissue and spinal cord after OEC transplantation.
Challenges
Despite the breadth of information presented, and although many of the suggestions in the initial review were addressed well, some points related to quantification and discussion of sex differences are not fully addressed in this revision.
(1) The request for quantification of OEC bridges is not fully addressed. We note that this revision includes the following statement (page 6): "We note, however, that such bridge formation is rare following a severe spinal cord injury in adult mammals." However, the title of the paper states that olfactory ensheathing cells promote neural repair and the abstract states that "OECs transplanted near the injury site modify the inhibitory glial scar and facilitate axon regeneration past the scar border and into the lesion." Statements such as these make it more crucial to include quantification of OEC bridges, because if single images are shown of remarkable, unusual bridges, but only one sentence acknowledges the low frequency of this occurrence, then this information taken together might present the wrong takeaway to readers.
Including some sort of quantification of bridging, whether it be the number of rats exhibiting bridges, the percentage area of OECs near a lesion site, or some other meaningful analysis, would add rigor and clarity to the manuscript.
(2) The additional discussion of sex differences in OEC bridging elaborates on the choice to study female rats, citing bladder challenges in male rats, but does not note salient clinical implications of this choice. Men account for ~80% of spinal cord injuries and likely also have worsened urinary tract issues, so it would be important to acknowledge this clinical fact and consider including males in future studies.
Author response:
The following is the authors’ response to the original reviews.
Joint Public Review:
Summary
This manuscript explores the transcriptomic identities of olfactory ensheathing cells (OECs), glial cells that support life-long axonal growth in olfactory neurons, as they relate to spinal cord injury repair. The authors show that transplantation of cultured, immunopurified rodent OECs at a spinal cord injury site can promote injury-bridging axonal regrowth. They then characterize these OECs using single-cell RNA sequencing, identifying five subtypes and proposing functional roles that include regeneration, wound healing, and cell-cell communication. They identify one progenitor OEC subpopulation and also report several other functionally relevant findings, notably, that OEC marker genes contain mixtures of other glial cell type markers (such as for Schwann cells and astrocytes), and that these cultured OECs produce and secrete Reelin, a regrowth-promoting protein that has been disputed as a gene product of OECs.
This manuscript offers an extensive, cell-level characterization of OECs, supporting their potential therapeutic value for spinal cord injury and suggesting potential underlying repair mechanisms. The authors use various approaches to validate their findings, providing interesting images that show the overlap between sprouting axons and transplanted OECs, and showing that OEC marker genes identified using single-cell RNA sequencing are present in vivo, in both olfactory bulb tissue and spinal cord after OEC transplantation.
Despite the breadth of information presented, however, further quantification of results and explanation of experimental approaches would be needed to support some of the authors' claims. Additionally, a more thorough discussion is needed to contextualize their findings relative to previous work.
(1) a. Important quantification is lacking for the data presented. For example, multiple figures include immunohistochemistry or immunocytochemistry data (Figures 1, 5, 6), but they are presented without accompanying measures like fractions of cells labeled or comparisons against controls.
We would like to clarify that the immunohistochemistry or immunocytochemistry data presented are meant to be qualitative rather than quantitative. The main purpose of the images is to show the presence or absence of markers of OEC subtypes rather than how much is present. That being said, in the revision we now add quantitative estimates of cell fractions for OECs along with other major cell types in Supplemental Table 1 and each OEC subtype marker in Supplemental Table 2.
b. As a result, for axons projecting via OEC bridges in Figure 1, it is unclear how common these bridges are in the presence or absence of OECs.
We note that the number of spinal cord transected rats with bridges of axons crossing the lesion core are extremely rare following a severe spinal cord injury in adult mammals. Our first example of axon bridging following a complete spinal cord transection followed by OEC transplants was reported in Thornton et al., (2018) and compared to an incomplete transection in a fibroblast-transplanted control in his Figure 4. That figure also appeared the cover of Experimental Neurology when the paper was published. Figure 1 in the current paper was from an independent experiment which replicated the previously observed rare bridge formation. We noted this in the revised manuscript.
Page 6: “We note, however, that such bridge formation is rare following a severe spinal cord injury in adult mammals.”
c. For Figure 6., it is unclear whether cells having an alternative OEC morphology coincide with progenitor OEC subtype marker genes to a statistically significant degree. (see top paragraph on page 11)
Franceschini & Barnett (1996) suggested that there were 2 distinct types of OECs that could be distinguished by their different morphology: one type resembling a Schwann cell and the other, an astrocyte. The purpose of Figure 6 is to determine if there is a link between our OEC subtypes based on scRNAseq with those previously described based on morphology alone (Franceschini and Barnett, 1996). There could be agreement between large, flat or small fusiform OECs morphological and their progenitor status, but it is not required that the two classification types would significantly overlap. Here we report the percentage of morphology-based cell subtypes that show expression of our OEC subtype markers to estimate the overlap between the two. Our results indicate the two types of OEC morphologies share a certain degree of overlap, a finding that indicates similarities as well as differences between the two classification methods.
In our results section we show that ~3/4ths of the Ki67-expressing OEC progenitor cells sampled were astrocyte-like, i.e., flat in shape and weakly Ngfrp75-labeled. The remaining ~1/4th of the Ki67-labeled OECs were fusiform in shape and expressed Ngfrp75 strongly. We feel that this is important to include as it is the only previous report of OB-OEC subtypes. The statistics of these results were in our original manuscript on page 11 and we further revise the text as follows:
Page 12: “To determine if the proliferative OECs differ in appearance from adult OECs, and whether there is concordance between our OEC subtypes based on gene expression markers and previously described morphology-based OEC subtyping (Franceschini & Barnett, 1996), we analyzed OECs identified with the anti-Ki67 nuclear marker and anti-Ngfrp75 (Figure 6g-h). Of the Ki67-positive OECs in our cultures, 24% ± 8% were strongly Ngfrp75-positive and spindle-shaped, whereas 76% ± 8% were flat and weakly Ngfrp75-labeled (n=4 cultures, p= 0.023). Here we show that a large percentage (~3/4ths) of proliferative OECs are characterized by large, flat morphology and weak Ngfrp75 expression resembling the previously described morphology-based astrocyte-like subtype. Our results indicate the two types of OEC classifications share a certain degree of overlap, indicating similarities but also differences between the two classification methods.”
d. Similar quantification is missing in other types of data such as Western blot images (Fig. 9) and OEC marker gene data (for which p-values are not reported; Table S2).
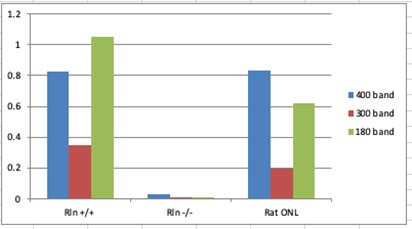
Response on Western blots: The Western blot signals shown in Figure 9 are from experiments that were designed to be qualitative rather than quantitative, by addressing the question, “Can we detect Reelin signals or not? in the different samples.” Both Western blots show that Reln+/+ mouse olfactory bulbs (d) or cortices (e) contain Reelin whereas Reln-/- samples do not and therefore provide positive and negative controls, respectively. The rat olfactory nerve layer (ONL, laminae I-II of olfactory bulb, d lane 1; e lane 3) contains mainly OECs wrapped around the axons of the olfactory sensory neurons that transmit olfactory signals into the olfactory bulb. To address your request for quantification, Dr. Khankan measured the density of the three isoforms of Reelin, 400 kD, 300 kD and 180 kD in Fig. 9e and normalized them against the GADPH control (37 kD). The graph below shows the normalized band density in arbitrary units on the Y-axis relative to the first 3 conditions, i.e., Reln+/+ and Reln-/- mouse cerebral cortices and rat Reln+/+ ONL. Because the conditioned medium was collected from tissue culture medium rather than cells or tissue, the GAPDH control was not present and therefore these data cannot be normalized in a similar analysis.
Author response image 1.
Response for OEC marker gene data: We now add new full supplementary Table S1 (for major cell types) and Table S2 (for OEC subtypes) to report statistical p values and adjusted p values, as well as additional statistics information including percent cell expressing a subtype marker in a given subtype versus in other subtypes.
e. The addition of quantitative measures and, where appropriate, statistical comparisons with p-values or other significance measures, would be important for supporting the authors' claims and more rigorously conveying the results.
As detailed in the above responses, we now add quantifications and statistics to support the claims and enhance the rigor of our analysis.
(2) a. Some aspects of the experimental design that are relevant to the interpretation of the results are not explained. For example, OECs appear to be collected from only female rats, but the potential implications of this factor are not discussed.
We added a short explanation in the Discussion and Methods section regarding why spinal cord injury studies are carried out on female rats.
Page 24, Discussion: “Due to the extensive urinary tract dysfunction in spinal cord transected rats, most studies prefer females as their short urethra facilitates daily manual bladder expression. Our study, therefore, was carried out only on adult female rats, so sex differences and the generalizability of our findings to adult male rats would require further investigation.”
Page 26, Methods: “Only females were used in order to match the sex of previous SCI studies conducted exclusively on female rats (Dixie, 2019; Khankan et al., 2016; Takeoka et al., 2011; Thornton et al., 2018). Following complete thoracic spinal cord transection, an adult rat is unable to urinate voluntarily and therefore urine must be manually “expressed” twice a day throughout the experiment. Females have a shorter urethra than males, and thus their bladders are easier to empty completely.”
b. Additionally, it is unclear from the manuscript to what degree immunopurified cells are OECs as opposed to other cell types. The antibody used to retain OECs, nerve growth factor receptor p75 (Ngfr-p75), can also be expressed by non-OEC olfactory bulb cell types including astrocytes [1-3]. The possible inclusion of Ngfr-p75-positive but non-OEC cell types in the OEC culture is not sufficiently addressed.
(a) Cragnolini, A.B. et al., Glia, (2009), doi: 10.1002/glia.20857.
(b) Vickland H. et al., Brain Res., (1991), doi: 10.1016/0006-8993(91)91659-O.
(c) Ung K. et al., Nat Commun., (2021), doi: 10.1038/s41467-021-25444-3.
Our OECs are dissected primarily from the olfactory nerve layer that is concentrated medially and ventrally around the olfactory bulb together with a small part of the glomerular layer (layer II). OECs are the only glia present in olfactory nerve layer. Thus, although it is possible that other cell types also express Ngfr-p75 as pointed out by the reviewer and in the references provided, our OEC dissection method severely limits the number of astrocytes that might be included in our cultures. We further provide additional evidence (see updated Figure 2d and the detailed responses to the next question) that our immunopanned OECs using our dissection method consistently express all classic OEC markers but do not consistently express the majority of classic markers for other glial cell types such as astrocytes or oligodendrocytes.
Such non-OEC cell types are also not distinguished in the analysis of single-cell RNA sequencing data (only microglia, fibroblasts, and OECs are identified; Figure 2). Thus, it is currently unclear whether results related to the OEC subtype may have been impacted by these experimental factors.
We need to clarify that when determining potential cell types in Figure 2, we compared our cell cluster marker genes against a broad array of cell types including astrocytes, oligodendrocytes and Schwann cells, but the gene overlap was only significant for microglia, fibroblasts, and OECs, which we labeled in new Figure 2d. We added more details in methods and results to clarify how we determined the cell types in Figure 2 (text added below). We did consider all the potential cell types that could have been present in our OEC cultures, including astrocytes. However, astrocyte or oligodendrocyte markers were not significantly enriched in the clusters, but markers for microglia, fibroblasts, and OECs were prominent in the cell clusters.
In the revised Figure 2d, we now illustrate that the OEC clusters not only express typical OEC markers, but also express a few but not all marker genes from other glial cells. We show the comparative data on markers for astrocytes, oligodendrocytes, and Schwann cells in Figure 2d in parallel with the marker genes for OECs, microglia, and fibroblasts. For each of the other glial cell types, there are some genes which overlap with OECs, and that is the reason why we identified OECs as hybrid glia.
Page 6, Results: “Based on previously reported cell type marker genes for fibroblasts and major glial cell types including OECs, astrocytes, oligodendrocytes, and microglia, we found elevated expression of OEC marker genes in clusters 2, 3 and 7, microglia marker genes in clusters 4, 6, and 7, and fibroblast marker genes in clusters 0, 1, and 5 (Figure 2d).”
Page 33, Methods: “Additional marker genes for fibroblasts and multiple glial cell types including astrocytes, oligodendrocytes, and microglia were also used to compare with those of the cell clusters.”
(3) The introduction, while well written, does not discuss studies showing no significant effect of OEC implantation after spinal cord injury. The discussion also fails to sufficiently acknowledge this variability in the efficacy of OEC implantation. This omission amplifies bias in the text, suggesting that OECs have significant effects that are not fully reflected in the literature. The introduction would need to be expanded to properly address the nuance suggested by the literature regarding the benefits of OECs after spinal cord injury. Additionally, in the discussion, relating the current study to previous work would help clarify how varying observations may relate to experimental or biological factors.
We appreciate the insightful comment and have now included information about the variability in OEC transplantation in previous studies in both the introduction and discussion sections. We discuss technical differences that lead to variability in the Introduction and how our findings could help interpret the variability in the Discussion.
Page 4-5: Text added to the Introduction: “The outcomes of OEC transplantation studies after spinal cord injury vary substantially in the literature due to many technical differences between their experimental designs. The source of OECs has a great impact on the outcome, with OB-OECs showing more promise than peripheral lamina propria-derived OECs, and purified, freshly-prepared OECs being required for optimal OEC survival. Other important variables include the severity of the injury (hemisection to complete spinal cord transection), the age of the spinal cord injured host (early postnatal versus adult), and OEC transplant strategies (delayed or acute transplantation, cell transplants with or without a matrix; Franssen et al., 2007). Franssen et al. (2007) evaluated studies that used only OECs as a transplant, and reported that 41 out of 56 studies showed positive effects, such as OEC stimulation of regeneration, positive interactions with the glial scar and remyelination of axons. More recent systematic reviews and meta-analyses on the effects of OEC transplantation following different spinal cord injury models reported that OECs significantly improved locomotor function (Watzlawick et al.2016; Nakjavan-Shahraki et al., 2018), but did not improve neuropathic pain (Nakjavan-Shahraki et al., 2018.)”
Pages 24-25: Discussion on OEC source variability “Extensive differences between OEC preparations contribute to the large variation in results from OEC treatments following spinal cord injury. This scRNA-seq study focused entirely on OB-OECs, and the next step would be to carry out similar studies on the peripheral, lamina-propria-derived OECs to discern the differences between these OEC populations. Such comparative studies using scRNA-seq will help define the underlying mechanisms and help resolve the variability in results from OEC-based therapy. Detailed studies of the composition of different OEC transplant types will contribute to identifying the most reparative cell transplantation treatments.”
Reviewer #1 (Recommendations For The Authors):
This is an extremely well-written and impactful series of experiments from a renowned leader in the field. The experimental questions are timely, with similar therapeutic approaches being prepared for clinical trial. The results address a gap that has persisted in the field for several decades and one that has been considered by many scientists long before technology existed to find answers. This highlights the importance of these experiments and the results reported here. With these things in mind, there are only a few minor factors that I have, that should be addressed to strengthen the paper.
We truly appreciate the positive evaluations from the reviewer!
Primary concerns
(1) Quantification of results: The authors report on the data with broad brush strokes, missing the opportunity to quantify results and strengthen the interpretations. For instance, when describing gene expression, what proportion of cells analyzed were expressing these genes? How did this compare with detectable levels of protein? Can the author draw correlations between data sets collected that could offer even more insight into the identities of the cells studied? There is also a missed opportunity to evaluate how transplantation into injured neural tissue might alter gene expression of the phenotypes identified prior to transplantation.
We appreciate these insightful comments and have added quantitative information and other relevant discussions in the revision. We now add Suppl Tables 1 (for major cell types including OECs, fibroblast, and microglia) and 2 (for OEC subtypes) to indicate the proportion of cells expressing each marker gene in each given cell cluster/subtype in the column. “Percentage of cells expressing the gene in the subtype/cell type” versus the proportion of cells expression the given marker genes in other cell types in the column “Percentage of cells expressing the gene in the other subtypes/cell types.” In the new supplementary tables, we report statistical p values and adjusted p values after multiple testing correction to indicate statistical significance.
Regarding the comparison with protein levels, we carried out immunohistochemistry experiments to confirm the proteins corresponding to OEC subtype markers. Our findings show that proteins for the gene markers can be detected, and thereby supports our sc-seq findings. However, the immunofluorescence only provides a qualitative measure of protein levels in situ, so we cannot perform a correlation analysis. This is something we plan to pursue in a follow-up study with measurable protein levels. We also discuss future directions to examine the genes and proteins in in vivo transplantation studies in the Discussion.
(2) Discussion and interpretation: Greater depth to interpretation and discussion of data and its impact on future work is needed. For example, on pages 20-21, the authors reflect briefly on why Reelin might be of interest (it could lead to Dab-1 expression), but why is that important? There are several instances like this where it would be useful for the authors to provide a little more insight into the potential impact of these data and interpretations.
We appreciate these valuable suggestions. We have revised our Results and Discussion sections to offer deeper insight and interpretation of the importance of the data, especially that for Reelin.
Page 17: Results: “In the canonical Reelin-signaling pathway, Reelin binds to the very-low-density lipoprotein receptor (Vldlr) and apolipoprotein E receptor 2 (ApoER2) and induces Src-mediated tyrosine phosphorylation of the intracellular adaptor protein Disabled-1 (Dab1). Both Reelin and Dab1 are highly expressed in embryos and contribute to correct neuronal positioning.”
Page 22-23, Discussion: “Reelin is a developmentally expressed protein detected in specific neurons, in addition to OECs and Schwann cells. The canonical Reelin-signaling pathway involves neuronal-secreted Reelin binding to Vldlr and ApoER2 receptors expressed on Dab1-labeled neurons. Following Reelin binding, Dab1 is phosphorylated by Src family kinases which initiates multiple downstream pathways. Very little is known, however, about Reelin secreted by glia. Panteri et al. (2006) reported that Schwann cells express low levels of Reelin in adults, and that it is upregulated following a peripheral nerve crush, as is reported above for many neurotrophic factors. Reelin loss in Schwann cells reduced the diameter of small myelinated axons but did not affect unmyelinated axons (Panteri et al., 2005). In the olfactory system, OECs ensheath the Dab1-labeled, unmyelinated axons of olfactory sensory neurons which are continuously generated and die throughout life. OEC transplantation following spinal cord injury would provide an exogenous source of Reelin that could phosphorylate Dab1-containing neurons or their axons. Dab1 is expressed at high levels in the axons of some projection neurons, such as the corticospinal pathway (Abadesco et al., 2014). Future experiments are needed to explore the function that glial-secreted Reelin may have on axonal regeneration.”
Minor concerns
(3) The authors reflect on the spontaneous glial bridge that develops in the repairing spinal cord of Zebrafish, but perhaps even more relevant is that this same phenomenon occurs in mammals as well if the spinal cord is injured during early development (opossum; Lane et al, EJN 2007). This should be considered and the statement that there is little regeneration in the mammalian spinal cord should be clarified.
We appreciate this insightful comment. We now add discussions of the axonal regeneration and bridging observed following severe spinal cord injury in young developing mouse and opossum spinal cords.
Page 23: “Adult mammals show little evidence of spontaneous axonal regeneration after a severe spinal cord injury in contrast to transected neonatal rats (Bregman, 1987; Bregman et al., 1993) and young postnatal opossums (Lane et al., 2007). In immature mammals, axons continue to project across or bridge the spinal cord transection site during development. Lower organisms such as fish, show even more evidence of regeneration following severe SCI. Mokalled et al. (2016) reported that glial secretion of Ctgfa/Ccn2 was both necessary and sufficient to stimulate a glial bridge for axon regeneration across the zebrafish transection site. Cells in the injury site that express Ctgf include ependymal cells, endothelial cells, and reactive astrocytes (Conrad et al., 2005; Mokalled et al., 2016; Schwab et al., 2001). Here we show that, although rare, Ctgf-positive OECs can contribute to glial bridge formation in adult rats. The most consistent finding among our severe SCI studies combined with OEC transplantation is the extent of remodeling of the injury site and axons growing into the inhibitory lesion site, together with OECs and astrocytes. The formation of a glial bridge across the injury was critical to the spontaneous axon generation seen in zebrafish (Mokalled et al., 2016) and likely contributed to the axon regeneration detected in our OEC transplanted, transected rats (Dixie, 2019; Khankan et al., 2016; Takeoka et al., 2011; Thornton et al., 2018).
Reviewer #2 (Recommendations For The Authors):
(1) The manuscript title and abstract must include the species and sex studied.
The title and abstract have been modified as suggested.
Page 1: “Olfactory ensheathing cells from adult female rats are hybrid glia that promote neural repair”
(2) OECs submitted for sequencing were like those about to be transplanted; however, the phenotype of the cells would likely change immediately and shift over time post-implantation. Please briefly address or discuss this point in the Discussion (or Results).
We have added this important discussion point.
Pages 23-24: Discussion: “We recognize that this study is a single snapshot of OEC gene expression derived from adult female rats before they are transplanted above and below the spinal cord transection site. We would expect the gene expression of transplanted OECs to change in each new environment, i.e. as they migrate into the injury site, integrate into the glial scar, and wrap around axons. Based on our past studies, OECs survived in an outbred Sprague-Dawley rat model for ~ 4 weeks (Khankan et al., 2016) and in an inbred Fischer 344 model for 5 months (Dixie, 2019). As spinal cord injury transplant procedures are further enhanced and OEC survival improves, these hybrid glial cells should be examined at multiple time points to better evaluate their proregenerative characteristics.”
(3) Page 12: Use of "monocytes" - the word "monocyte" implies a circulating, undifferentiated innate immune cell. This should not be used interchangeably with macrophage or microglia.
We agree and now refer to microglia or macrophages depending on the context. We did leave the term monocyte in Table 3 if these cells were found in a top 20 gene reported in the references.
(4) Page 12: "We now show that these unique monocytes reported between the bundles of olfactory axons surrounded by OECs (Smithson & Kawaja, 2010), are in fact, a distinct subtype of OECs."
Is it possible to conclude that these cells are a "distinct subtype of OECs?" Perhaps these cells are a hybrid between microglia/macrophages and OECs? This is speculative, so should be worded more carefully - especially in the Results section. Please clarify, dampen conclusions, and/or better justify the wording here.
We agree and have modified the entire paragraph to dampen and more carefully explain our conclusions. We also added an additional observation that strengthens the relationship between OECs and microglial/macrophages.
Page 12, Results: Additional observation: “In fact, all top 20 genes in cluster 3 are expressed in microglia, macrophages, and/or monocytes (Suppl. Table 3).”
Page 13, Results: The statement referenced in your review was deleted and we wrote the following: “Smithson and Kawaja (2010) identified unique microglial/macrophages that immunolabeled with Iba-1 (Aif1) and Annexin A3 (Anxa3) in the olfactory nerve and outer nerve layer of the olfactory bulb. These authors proposed that Iba1-Anxa3 double-labeled cells were a distinct population of microglia/macrophages that protected the olfactory system against viral invasion into the cranial cavity. Based on our scRNA-seq data we offer an alternative interpretation that at least some of these Iba-1-Anxa3 cells may be a hybrid OEC-microglial cell type. Supporting this interpretation, there are a number of reports that suggest OECs frequently function as phagocytes (e.g., Khankan et al., 2016; Nazareth et al., 2020; Su et al. 2013).”
(5) Page 13: "Pseudotime trajectory analysis, a widely used approach to predict cell plasticity and lineages based on scRNA-seq data, suggests that there are potential transitions between specific OEC subclusters." This is interesting but is somewhat unclear. Please add one more sentence to aid the reader's understanding regarding how this analysis is performed.
Thank you for your valuable feedback. We have revised the text for clarity as follows:
Page 14, Results: “We performed pseudotime trajectory analysis using the Slingshot algorithm to infer lineage trajectories, cell plasticity and lineages by ordering cells in pseudotime based on their transcriptional progression reflected in scRNA-seq data. Transcriptional progression refers to the changes in gene expression profiles of cells as they undergo differentiation or transition through different states. The trajectory analysis results suggest that there are potential transitions between specific OEC subclusters.”
(6) The authors could discuss potential reasons for variability in OEC treatment results after spinal cord injury between studies and labs. How might sequencing results here inform the debate about whether OECs are helpful or not?
In response to the Public Review, we added discussions about the variability in OEC treatments between studies in both the Introduction and Discussion, and these comments are copied on pages 6-7 of this document. In the Discussion we included a statement about how the current findings may inform the debate on OECs.
(7) Discussion: please add a discussion of limitations and future directions that addresses the following points:
a) Please add one sentence on the lack of studying sex differences - only females were studied here.
b) There is no correlation or modulation of any target genes, so all results here are correlative.
c) Please add a brief paragraph with future directions for the field, including acknowledgment that the role of OECs in repair after SCI is not fully resolved and that future studies might consider targeting some of the specific pathways described herein.
d) Which pathways and OEC subpopulations likely best support repair, and how might these be reinforced or better maintained in the SCI environment? If not known, what are the next steps for identifying the most reparative OEC subtype?
Thank you for the valuable suggestions. We have added these to the discussion as detailed below.
Pages 23-25, Discussion:
“Limitations of these OEC scRNA-Seq studies”
“We recognize that this study is a single snapshot of OEC gene expression derived from adult female rats before they are transplanted above and below the spinal cord transection site. We would expect the gene expression of transplanted OECs to change in each new environment, i.e. as they migrate into the injury site, integrate into the glial scar, and wrap around axons. Based on our past studies, OECs survived in an outbred Sprague-Dawley rat model for ~ 4 weeks (Khankan et al., 2016) and in an inbred Fischer 344 model for 5 months (Dixie, 2019). As spinal cord injury transplant procedures are further enhanced and OEC survival improves, these hybrid glial cells should be examined at multiple time points to better evaluate their proregenerative characteristics.”
“Due to the extensive urinary tract dysfunction in spinal cord transected rats, most studies are conducted on females as their short urethra facilitates daily manual bladder expression. Our study was carried out only on adult female rats, so sex differences and the generalizability of our findings to adult male rats would require further investigation. We also did not modulate any of the genes or proteins in the identified OEC subtypes to test their causal and functional roles, thus our findings remain correlative in the current study. Future gene/protein modulation studies are necessary to understand the functional roles of the individual OEC subtypes in the context of their reparative functions to determine which pathways and subtypes are more critical and can be enhanced for neural repair. Our current findings build the foundation for these future studies to help resolve the role of OECs in spinal cord injury repair.”
“Extensive differences between OEC preparations contribute to the large variation in results from OEC treatments following spinal cord injury. This scRNA-seq study focused entirely on OB-OECs, and the next step would be to carry out similar studies on the peripheral, lamina-propria-derived OECs to discern the differences between the two OEC populations. Such comparative studies using scRNA-seq will help define the underlying mechanisms and resolve the variability in results from OEC-based therapy. Detailed studies of the composition of different OEC transplant types will contribute to identifying the most reparative cell transplantation treatments.”
(8) Figure 6: What is the major point of this figure and its related immunocytochemistry? Please clarify.
Franceschini & Barnett (1996) suggested that there were 2 distinct types of OECs that could be distinguished by their different morphology: One type resembling a Schwann cell and the other, an astrocyte. The purpose of Figure 6 is to determine if there is a link between our scRNA-seq-based OEC subtypes with those previously described based on morphology alone (Franceschini and Barnett, 1996). In our results section we show that ~3/4ths of the OECs sampled that were Ki67+ progenitor cells and were astrocyte-like, i.e., flat in shape and weakly Ngfrp75-labeled. The remainder were Schwann cell-like, fusiform in shape and strongly Ngfrp75-labeled. Our results indicate the two types of OEC classifications share certain degrees of overlap, indicating similarities but also differences between the different classification methods.
(9) Figure 9, caption: "OEC whole cell lysates (WCL; lanes: 4, 6, and 8), and OEC conditioned medium (CM; lanes: 5 and 7)." This statement is unclear - please clarify the result here.
We added clarification to the legend for Figure 9d.
Page 50: (d) “Western blot confirms the expression of Reelin in rat olfactory nerve layer I and layer II (ONL; lane 1 of western blot). Reln+/+ and Reln-/- mouse olfactory bulbs were used as positive and negative controls, respectively (lanes: 2 and 3). Reelin that was synthesized by cultured OECs was found in whole cell lysates (WCL; lanes: 4, 6, and 8), whereas Reelin that was secreted by cultured OECs into tissue culture medium was measured in the OEC “conditioned medium” (CM; lanes: 5 and 7). GAPDH was the loading control for tissue homogenates (lanes 1-4, 6, 8).”
(10) Methods: A Cat. No. for all antibodies and key supplies should be included.
Response: All of the antibody information in the revised version is in Suppl. Table 4. Information for other key supplies is included in the extensive methods section.
(11) Methods: How was primary antibody specificity validated for less-used antibodies? Background staining can be a major issue after SCI; e.g., with the CTGF antibody used in Figure 5.
The spinal cord section shown in Figure 5 was compared to sections from the same SCI cohort that had been injected with control cells, i.e. skin fibroblasts. We have used the first two antibodies (anti-Glial fibrillary acidic protein and anti-Green fluorescent protein) for many years so only the CTGF was a “less-used antibody.” Our strategy for working with “less-used” or “newly-purchased” antibodies was as follows.
First, we studied the literature to find the best antibodies for neuronal tissue. Many of the images in Figure 7 were generated with antibodies purchased just for this study. Our goal was to characterize them on normal adult lamina propria and olfactory bulb tissues rather than in the injured spinal cord where background can be an issue. In the olfactory bulb we examined the olfactory nerve layer where OECs are concentrated and then examined the olfactory epithelium, lamina propria, and the deep layers of the olfactory bulb to find regions without immunolabel. As described above, we tested anti-CTGF antibodies in SCI sections implanted with skin fibroblasts controls when conducting experiments for CTGF in sections with OECs. New antibodies were tested at multiple concentrations and we tried different immunocytochemical techniques. Anti-CTFG is expressed by several different cell types, but expression is low in most of the areas above and below the injury site. Despite our success with many “newly-purchased” antibodies there were at least 4 of them that we were never able obtain specific labeling.
(12) Will the data (especially the sequencing data) be shared publicly?
The data has been uploaded to and shared via the public data repository GEO. Data availability is stated on the title page of this manuscript.