Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
TMC7 knockout mice were generated by the authors and the phenotype was analyzed. They found that Tmc7 is localized to Golgi and is needed for acrosome biogenesis.
Strengths:
The phenotype of infertility is clear, and the results of TMC7 localization and the failed acrosome formation are highly reliable. In this respect, they made a significant discovery regarding spermatogenesis.
Weaknesses:
There are also some concerns, which are mainly related to the molecular function of TMC7 and Figure 5.
(1) It is understandable that TMC7 exhibits some channel activity in the Golgi and somehow affects luminal pH or Ca2+, leading to the failure of acrosome formation. On the other hand, since they are conducting the pH and calcium imaging from the cytoplasm, I do not think that the effect of TMC7 channel function in Golgi is detectable with their methods.
We agree with the reviewer that there are no direct evidences showing the effect of TMC7 channel function in Golgi. We have changed the description in the revised manuscript.
(2) Rather, it is more likely that they are detecting apoptotic cells that have no longer normal ion homeostasis.
We thank the reviewer for raising this concern. We apologize for not labeling the postnatal stage in original Figure 5. We measured intracellular Ca2+, pH and ROS in PD30 testes (revised Fig. S6a-c), no apoptotic cells were observed at this stage (revised Fig. S6e, f). Apoptotic cells were found in the seminiferous tubules and cauda epididymis of 9-week-old Tmc7–/– mice (revised Fig. 5e-f). We have included TUNEL data in testis of PD21, PD30 and 9-week-old mice (revised Fig. 5e, f and Fig. S6e, f). In accordance with our findings, Tmc1 mutation has also been shown to result in reduced Ca2+ permeability, thus triggering hair cell apoptosis (Fettiplace, R, PNAS. 2022) [1].
(3) Another concern is that n is only 3 for these imaging experiments.
As suggested by the reviewer, more replicates were included in imaging experiments.
Reviewer #2 (Public Review):
Summary:
This study presents a significant finding that enhances our understanding of spermatogenesis. TMC7 belongs to a family of transmembrane channel-like proteins (TMC1-8), primarily known for their role in the ear. Mutations to TMC1/2 are linked to deafness in humans and mice and were originally characterized as auditory mechanosensitive ion channels. However, the function of the other TMC family members remains poorly characterized. In this study, the authors begin to elucidate the function of TMC7 in acrosome biogenesis during spermatogenesis. Through analysis of transcriptomics datasets, they identify TMC7 as a transmembrane channel-like protein with elevated transcript levels in round spermatids in both mouse and human testis. They then generate Tmc7-/- mice and find that male mice exhibit smaller testes and complete infertility. Examination of different developmental stages reveals spermatogenesis defects, including reduced sperm count, elongated spermatids, and large vacuoles. Additionally, abnormal acrosome morphology is observed beginning at the early-stage Golgi phase, indicating TMC7's involvement in proacrosomal vesicle trafficking and fusion. They observed localization of TMC7 in the cis-Golgi and suggest that its presence is required for maintaining Golgi integrity, with Tmc7-/- leading to reduced intracellular Ca2+, elevated pH, and increased ROS levels, likely resulting in spermatid apoptosis. Overall, the work delineates a new function of TMC7 in spermatogenesis and the authors suggest that its ion channel activity is likely important for Golgi homeostasis. This work is of significant interest to the community and is of high quality.
Strengths:
The biggest strength of the paper is the phenotypic characterization of the TMC7-/- mouse model, which has clear acrosome biogenesis/spermatogenesis defects. This is the main claim of the paper and it is supported by the data that are presented.
Weaknesses:
The claim is that TMC7 functions as an ion channel. It is reasonable to assume this given what has been previously published on the more well-characterized TMCs (TMC1/2), but the data supporting this is preliminary here, and more needs to be done to solidify this hypothesis. The authors are careful in their interpretation and present this merely as a hypothesis supporting this idea.
We appreciate the insightful comment. It is indeed a limitation of our study that we lack strong evidences to support that TMC7 functions as an ion channel. We have planned to conduct cellular electrophysiology in GC-1 cells heterologous expression of TMC7. However, TMC7 was trapped in the endoplasmic reticulum like TMC1 and TMC2 (Yu X, PNAS. 2020)[2], and failed to localize to the Golgi. According to the reviewer’s suggestion, we have made careful and more detailed interpretation the molecular function of TMC7 in the revised manuscript.
Reviewer #3 (Public Review):
Summary:
In this study, Wang et al. have demonstrated that TMC7, a testis-enriched multipass transmembrane protein, is essential for male reproduction in mice. Tmc7 KO male mice are sterile due to reduced sperm count and abnormal sperm morphology. TMC7 co-localizes with GM130, a cis-Golgi marker, in round spermatids. The absence of TMC7 results in reduced levels of Golgi proteins, elevated abundance of ER stress markers, as well as changes of Ca2+ and pH levels in the KO testis. However, further confirmation is required because the analyses were performed with whole testis samples in spite of the differences in the germ cell composition in WT and KO testis. In addition, the causal relationships between the reported anomalies await thorough interrogation.
Strengths:
The microscopic images are of great quality, all figures are properly arranged, and the entire manuscript is very easy to follow.
Weaknesses:
(1) Tmc7 KO male mice show multiple anomalies in sperm production and morphogenesis, such as reduced sperm count, abnormal sperm head, and deformed midpiece. Thus, it is confusing that the authors focused solely on impaired acrosome biogenesis.
We are grateful to your comments and suggestions. We agree and have added these defects in spermiogenesis of Tmc7–/– mice in the abstract and discussion sections of revised manuscript.
(2) Further investigations are warranted to determine whether the abnormalities reported in this manuscript (e.g., changes in protein, Ca2+, and pH levels) are directly associated with the molecular function of TMC7 or are the byproducts of partially arrested spermiogenesis. Please find additional comments in "Recommendations for the authors".
Thank you for raising this concern. Per your comments, we have included data of intracellular Ca2+, pH and ROS in PD21 testes. The intracellular homeostasis was impaired as early as PD21, indicating TMC7 depletion impairs cellular homeostasis which in turn results in arrested spermiogenesis.
Recommendations for the authors:
Reviewing Editor (Recommendations For The Authors):
As noted by all three reviewers, current flow cytometry data does not necessarily support the 'ion channel' hypothesis, thus the phenotypic analysis is compelling but the molecular mechanism of how TMC7 facilitates acrosome biogenesis remains incomplete. It is highly recommended for the authors to at least discuss or test alternative hypotheses (as reviewer #2 suggested) such as the possibility of acting as 'lipid scramblase'. Also, the authors need to provide further explanation for other morphological defects if TMC7 is truly a functional ion channel in Golgi (and thus later at acrosome), which is also related to the key question of whether TMC7 is a functional ion channel.
We thank the reviewing editor for the comments and suggestions. We agree that our study lack strong evidences to support that TMC7 functions as an ion channel. We have discussed the possibility of TMC7 acting as 'lipid scramblase' as suggested. We have also included data of intracellular Ca2+, pH and ROS in PD21, PD30 testes.
Indeed, Tmc7–/– mice exhibits other defects including abnormal head morphology and disorganized mitochondrial sheaths. As TMC7 is localized to the cis-Golgi apparatus and is required for maintaining Golgi integrity. Previous studies on Golgi localized proteins including GOPC (Yao R, PNAS. 2002)[2], HRB (Kang-Decker N. Science. 2001)[3] and PICK1(Xiao N, JCI. 2009)[4] exhibit similar defects in spermiogenesis with Tmc7–/– mice. It is possible that defects morphologies in Tmc7–/– mice might be due to impaired function of Golgi.
Reviewer #1 (Recommendations For The Authors):
(1) The authors should provide more details about the imaging experiments using FACS. Since they only describe catalog numbers (Beyotime, S1056, S1006, S0033S) for imaging reagents, it is not immediately clear what reagents they actually used. Since they used Fluo3, BCECF, and DCFH, it would be better to mention their names.
Thanks. We have provided more detailed antibody information as suggested.
(2) I am also concerned that in the FACS there is no information at all about laser wavelength and filter properties. This is especially important for BCECF because the wavelength spectrum changes with pH. Also, if there are any positive controls for these imaging reagents, such as ionophores, it would be more convincing to include them.
Thank you for your comment. Excitation wavelength is 488nm for detecting Ca2+, pH and ROS in FACS. BCECF is the most popular pH probe to monitor cellular pH and the reagent from Beyotime (S1006) has been used by other studies (Chen S, Blood. 2016)[5], (Liu H, Cell Death Dis. 2022)[6]. To make the results more reliable, we have repeated these experiments in PD21 testes (revised Figure 5a-c). No positive controls for these reagents were used in our experiments.
(3) As noted above, it is better to avoid directly linking the cell's abnormal ion homeostasis to TMC7 ion channel function in the text. The discussion should be changed to emphasize that the TMC7-deficient cells are apoptotic and that these physiological phenomena are occurring as a side effect of this apoptosis.
Thank you for raising this concern. We agree with the reviewer that there are no direct evidences showing the effect of TMC7 channel function in Golgi and we have changed the description in the revised manuscript.
We performed new experiment to measure apoptosis and intracellular Ca2+, pH and ROS in PD21 testes. No apoptotic cells were observed at this stage. However, impaired cellular homeostasis was still found in testis of PD21 Tmc7-/- mice. These data suggest that TMC7 depletion impairs cellular homeostasis and hence induces spermatid apoptosis.
(4) While I understand that it appears to be difficult to experimentally verify the ion channel function of TMC7, it may be supportive to compare its amino acid sequence and/or 3D predicted structure with that of TMC1/2. Including a supplemental figure for this purpose would emphasize the possibility that TMC7 functions as an ion channel.
We thank the reviewer for making this great suggestion. We compared the amino acid sequence and structure of TMC1, TMC2 with TMC7 respectively. TMC1 had 81% sequence similarity with TMC7 and the RMSD (Root Mean Square Deviation) was 3.079. TMC2 had 82% sequence similarity with TMC7, the RMSD was 2.176. These data suggest that TMC7 has similar amino acid sequence and predicted structure with TMC1/2 and might functions as an ion channel. We have included the predicted structures in revised Fig. S7.
Author response image 1.
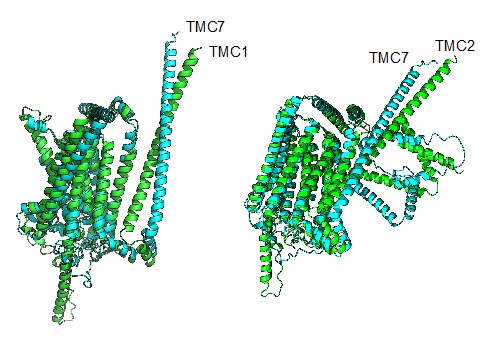
Reviewer #2 (Recommendations For The Authors):
I do not have any experimental comments or concerns to address, but I do ask that the authors consider an alternative hypothesis. Based on prior data demonstrating that TMC1 is a mechanosensitive ion channel, the authors reasonably assume that TMC7 may also function as an ion channel. Although the authors observe alterations in cytosolic Ca2+ and pH upon loss of TMC7 by flow cytometry, which begins to support this hypothesis, these data do not directly demonstrate ion channel activity.
I was wondering if the authors had considered whether TMC7 could also function as a lipid scramblase. TMC1 has also been proposed to function as a Ca2+-inhibited scramblase, where knockout of TMC1 leads to a loss of phosphatidylserine (PS) exposure and membrane blebbing at the apical region of hair cells (Ballesteros, A. and Swartz, K., Science Advances, 2022). Furthermore, TMC proteins are structurally related to the Anoctamin/TMEM16 family of chloride channels and lipid scramblases, where TMEM16A-B are bona fide Ca2+-activated chloride channels, and TMEM16C-H are characterized as Ca2+-dependent scramblases. Based on their structural similarity and the observation that TMC1 may also exhibit lipid scrambling properties based on the PS exposure, I wonder if the authors may have data that support a TMC7 scramblase hypothesis. I was intrigued by this idea, especially given the authors' observations of large vacuoles in the seminiferous tubules and cauda epididymis and the vesicle accumulation phenotype in their TEM data. Incorporating this hypothesis into the discussion section, at minimum, could provide a valuable perspective, and this line of thought may lead to interesting data interpretation throughout the paper.
We thank the reviewer for the valuable suggestion. We have discussed the possibility of TMC7 acting as 'lipid scramblase' as suggested.
Reviewer #3 (Recommendations For The Authors):
(1) Gene symbols should be italicized, and protein symbols should be capitalized.
Thanks. We have made changes to the manuscript as recommended.
(2) Tmc7 KO males show reduced sperm count, which alters the germ cell composition in the testis (Figure 2g). Thus, it is inappropriate to compare protein levels using whole testis lysates (Figure 3e, 4h, 5d, 5f). Instead, the same immunoblotting analyses could be done with purified round spermatids or 3-wk-old testis. Likewise, the significance of the intracellular Ca2+ and pH measurements is potentially diminished by the differences in the germ cell composition in WT and KO mice.
We appreciate this constructive suggestion. We agree with the reviewer that whole testis lysates diminished the differences between WT and _Tmc7-/-_mice. However, we are unable purify round spermatids due to the lack of specific markers.
(3) Figures 2i, 2j: How sperm motility was measured should be specified in the Methods.
We thank you for your significant reminding and have added sperm motility assessment in Methods section.
(4) Figure 4g: It does not make sense to compare the fluorescence intensity of these proteins without making sure that the seminiferous tubules are in the same stage. As shown in Figures S5a and S5b, TMC7 exhibits varied abundance in spermatids at different steps.
We thank the reviewer for the insightful comment. We have replaced images in the same stage seminiferous tubules and compared the fluorescence intensity of new images as suggested.
(5) Figure 4h: How were the band intensities measured? The third band from the left is visually stronger than the first one, but it does not seem to be so according to the column graph. The reviewer measured the intensity of GRASP65 bands relative to alpha-tubulin by ImageJ and obtained relative intensities of 0.35, 0.87, 0.6, and 0.08 for the bands from left to right. Additional replicates of the western blots should be included in the supplementary figures.
Thank you for this insightful comment. The density and size of the blots were quantified by Image J. We have checked the first band from the left of GRASP65 and it seems that the protein was not fully transferred onto the PVDF membrane. We have performed new experiments and replaced the original bands (Revised Fig. 4h). Additional replicates of the western blots have been included in revised Fig. S8.
(6) Figures 5a, 5b: Based on the observation of abnormal intracellular Ca2+ and pH levels in the KO germ cells, the authors concluded that TMC7 maintains the homeostasis of Golgi pH and ion (Lines 223-224, 263-264). However, intracellular Ca2+ and pH levels do not directly reflect those in the Golgi apparatus.
We thank the reviewer for this important comment. We agree and have changed “Golgi” to “intracellular” as suggested.
(7) Figure 5c: ROS is produced during apoptosis. Thus, it is not appropriate to conclude that the increased ROS levels in Tmc7 KO germ cells lead to apoptosis.
According to the reviewer’s comment, we measured ROS and apoptosis in testis of PD21 and PD30 mice. ROS levels were increased, but no apoptotic cells were observed in testis of PD21 and PD30 Tmc7–/– mice. Apoptotic cells were observed in testis of 9-week-old Tmc7–/– mice (Revised Fig. 5e-f). These data suggest that TMC7 depletion results in the accumulation of ROS, thereby leads to apoptosis.
(1) Fettiplace, R., D.N. Furness, and M. Beurg, The conductance and organization of the TMC1-containing mechanotransducer channel complex in auditory hair cells. Proc Natl Acad Sci U S A, 2022. 119(41): p. e2210849119.
(2) Yu, X., et al., Deafness mutation D572N of TMC1 destabilizes TMC1 expression by disrupting LHFPL5 binding. Proc Natl Acad Sci U S A, 2020. 117(47): p. 29894-29903.
(3) Kang-Decker, N., et al., Lack of acrosome formation in Hrb-deficient mice. Science, 2001. 294(5546): p. 1531-3.
(4) Xiao, N., et al., PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest, 2009. 119(4): p. 802-12.
(5) Chen, S., et al., Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood, 2016. 127(8): p. 1024-35.
(6) Liu, H., et al., PRMT5 critically mediates TMAO-induced inflammatory response in vascular smooth muscle cells. Cell Death Dis, 2022. 13(4): p. 299.




