Abstract
Mutational activation of KRAS occurs commonly in lung carcinogenesis and, with the recent FDA approval of covalent inhibitors of KRASG12C such as sotorasib or adagrasib, KRAS oncoproteins are important pharmacological targets in non-small cell lung cancer (NSCLC). However, not all KRASG12C-driven NSCLCs respond to these inhibitors, and the emergence of drug resistance in those patients that do respond can be rapid and pleiotropic. Hence, based on a backbone of covalent inhibition of KRASG12C, efforts are underway to develop effective combination therapies. Here we report that inhibition of KRASG12C signaling increases autophagy in KRASG12C expressing lung cancer cells. Moreover, the combination of DCC-3116, a selective ULK1/2 inhibitor, plus sotorasib displays cooperative/synergistic suppression of human KRASG12C-driven lung cancer cell proliferation in vitro and superior tumor control in vivo. Additionally, in genetically engineered mouse models of KRASG12C-driven NSCLC, inhibition of either KRASG12C or ULK1/2 decreases tumor burden and increases mouse survival. Consequently, these data suggest that ULK1/2-mediated autophagy is a pharmacologically actionable cytoprotective stress response to inhibition of KRASG12C in lung cancer.
Introduction
Lung adenocarcinoma (LUAD) is responsible for ∼50,000 deaths/year in the USA. Amongst the most common genetic drivers of LUAD are mutations in KRAS that encode KRAS-4A and -4B oncoproteins among which the substitution of cysteine for glycine at codon 12 (KRASG12C) is the most common mutation and is associated with exposure to tobacco smoke1,2. For many years, RAS (H-, K- or N) oncoproteins were considered undruggable until the seminal work of Ostrem et al. that demonstrated the feasibility of developing covalent inhibitors of KRASG12C that bind into the Switch II pocket of KRASG12C-GDP and derivatize the reactive thiol of cysteine 12 3–5.
We and others have previously reported that inhibition of RAS>RAF>MEK>ERK signaling in melanoma or pancreatic cancer cells elicits increased autophagy, an intracellular macromolecule and organelle recycling mechanism, through the LKB1>AMPK>ULK1 signaling axis6–9. Liver kinase B1 (LKB1) activates AMP kinases in response to cellular nutrient changes to maintain homeostasis10. However, induction of autophagy has also been reported in response to MEK inhibition in models of KRASG12D-driven lung cancer with loss of LKB1 expression11, suggesting that LKB1 is dispensable for increases in autophagy in lung cancer. LKB1 silencing is frequently observed in patients with KRAS-driven lung cancer, and such patients exhibit decreased overall survival and are more refractory to current therapeutic treatments than in LKB1 proficient KRAS-driven lung cancers12,13. With the advent of FDA-approved covalent KRASG12C inhibitors, it is imperative to investigate if autophagy occurs following inhibition of KRASG12C signaling, especially since MEK inhibitors offer little clinical benefit accompanied by toxicity to lung cancer patients14. This prompted us to explore if treatment with sotorasib leads to an increase in autophagy in KRASG12C mutant lung cancer cells with and without the expression of LKB1.
At present, the only FDA-approved therapeutics that inhibit autophagy are the 4-amino-quinolone lysosomal inhibitors chloroquine (CQ) and hydroxychloroquine (HCQ)15. Originally approved for treating malaria, HCQ acts by inhibiting Toll-like receptors16. It was also reported that HCQ inhibits acidification of the lysosome, thereby inhibiting auto-lysosome activity, although the precise mechanism of this remains obscure17–19. Clinically, high concentrations of HCQ are required to achieve modest inhibition of autophagy in patients, suggesting that the inhibitory potency of these compounds may limit clinical responses. Indeed, co-targeting lysosomal function in combination with RAS-driven signaling has recently been tested in RAS-driven cancers in several clinical trials8,9,20 (clinicaltrials.gov: NCT04214418, NCT04132505, NCT04386057, NCT04145297). However, there is a lack of clinical investigation on targeting autophagy with selective autophagy inhibitors in patients.
Autophagy is a complex intracellular recycling process important for maintaining cellular homeostasis under stressed conditions. The genetics and biochemistry of autophagy, first revealed in yeast21, indicate numerous points of regulation of which the initiation and formation of the autophagosome, as well as the fusion of the autophagosome to the lysosome are of key importance.21,22 ULK1/2 are protein serine/threonine kinases that form a complex with FIP200, ATG13 and ATG101, which integrates upstream signals from mTORC1 and AMPK energy-sensing pathways22. Upon activation, ULK1/2 phosphorylate a number of substrates including ATG13 (at serine 318, pS318) to initiate the formation of autophagosomes22. There remains interest in the development of selective inhibitors of autophagy for use in a number of disease indications. 23 DCC-3116 is one such “switch-control” ULK1/2 inhibitor, which is currently in Phase 1/2 clinical trials either as a monotherapy or in combination with inhibitors of KRASG12C, MEK1/2 or EGFR (NCT04892017 & NCT05957367).
Here, we test the anti-tumor effects of DCC-3116 against preclinical models of KRASG12C-driven lung cancer either alone or in combination with sotorasib. We observed that cultured KRASG12C-driven lung cancer cells increase autophagy in response to inhibition of KRASG12C>RAF>MEK>ERK signaling. In addition, combined inhibition of KRASG12C plus ULK1/2 leads to decreased cell proliferation and tumor growth. Moreover, using GEM models of ether KRASG12C/TP53R172H- or KRASG12C/LKB1Null-driven lung cancer24, we demonstrate that LKB1 is dispensable for increases in autophagy following KRASG12C inhibition. Furthermore, LKB1 silencing diminishes the sensitivity of KRASG12C/LKB1Null-driven lung cancer perhaps through the emergence of mixed adenosquamous cell carcinomas and mucinous adenocarcinomas. Adenosquamous carcinomas are observed in up to 4% of lung cancers and the emergence of these tumors is observed as a resistance mechanism to current therapeutics in lung cancer patients25. Patients with ASC tend to exhibit substantially decreased overall survival compared to patients with adenocarcinomas or squamous carcinomas26. These data suggest that KRASG12C mutant lung tumors with adenosquamous pathology may not respond to KRASG12C or ULK inhibition. Clinical acquired resistance to sotorasib and adagrasib is emerging in KRASG12C mutant lung cancer patients27–33. Here, we demonstrate that KRASG12C/LKB1Null-driven lung cancer cells that acquire resistance to sotorasib increase expression of RAS>RAF>MEK>ERK signaling, and no longer increase autophagy after sotorasib treatment. These cells do induce autophagy after MEK1/2 inhibition and decrease cellular proliferation and viability after treatment with trametinib, suggesting that sotorasib-resistant cells are still vulnerable to downstream KRASG12C signaling inhibition.
Results
Human KRASG12C-driven lung cancer cells are sensitive to co-inhibition of KRASG12C and ULK1/2
To determine if direct inhibition of KRASG12C in lung cancer cells influences autophagy, human KRASG12X-driven lung cancer cell lines were engineered to express a chimeric fluorescent autophagy reporter (FAR) comprised of mCherry fused to EGFP fused to LC38,34. LC3 targets the chimeric FAR to the autophagosome where the pH sensitive EGFP moiety is quenched in the acidic environment of the autolysosome (pH<5). Hence, an increase in the ratio of mCherry:EGFP fluorescence is indicative of increased autophagy8,34. All KRASG12C-driven lung cancer cells displayed dose-dependent increases in autophagy following sotorasib treatment as indicated by an increase in the mCherry:EGFP ratio detected in live cells (Figs. 1A-C, Figs. S1A-D). Since sotorasib is reported to bind to >300 proteins, we ruled out non-specific effects of sotorasib on autophagy, by treating a KRASG12V-driven NSCLC cell line (COR-L23) with sotorasib, which failed to show any change in mCherry:EGFP fluorescence (Fig. S1E) 35. Moreover, as predicted by previous experiments, all KRASG12X-driven NSCLC cell lines displayed a dose-dependent increase in autophagy following treatment with trametinib, a MEK1/2 inhibitor (Figs. S1F-J)8.

Human KRASG12C-driven lung cancer cells are sensitive to co-inhibition of KRASG12C and ULK1/2.
A-C, Human KRASG12C-driven cell lines NCI-H2122 (A), Calu-1 (B) and NCI-H358 (C) increase autophagy as assessed by mCherry-EGFP-LC3 reporter after 48 hours of sotorasib treatment and decrease autophagy after 48h hours of DCC-3116 treatment. Red=high autophagy, Yellow= medium autophagy, Green= low autophagy. Statistical significance was determined by comparing autophagic flux levels to DMSO control and an ordinary one-way ANOVA with Dunnett’s multiple comparisons was used. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=9
D-F, Quantification of percent confluence of human KRASG12C-driven cell lines at 72 hours post-drug treatment. Statistical significance was determined by an ordinary one-way ANOVA. Ns= not significant,*p<0.05 **p<0.01 ***p<0.001 ****p<0.0001.N=3
G- I, In vitro synergy assay of human KRASG12C-driven cell lines using the Loewe method after 72 hours of treatment. N=3
J-L, Tumor volume measured over 28 days of treatment in mice inoculated with NCI-H2122 (J), Calu-1
(K) and NCI-H358 (L) cells. Vehicle and sotorasib were administered once daily via oral gavage and DCC-3116 was formulated in the chow. Statistical significance was determined by an ordinary one-way
ANOVA compared to vehicle treated tumors. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=4-5 mice per treatment.
Next, we tested the effects of DCC-3116 on autophagy in KRASG12C-driven NSCLC cell lines. As anticipated, DCC-3116 inhibited both basal and sotorasib-induced autophagy in all KRASG12C-driven cell lines as assessed using the FAR (Figs. 1A-C). In addition, DCC-3116 treatment, either alone or in combination with sotorasib, led to decreased pS318-ATG13, one of ULK1’s immediate downstream substrate (Figs. S2A-C). To test if DCC-3116 might show additive or synergistic anti-proliferative effects when combined with sotorasib, human KRASG12C-driven NSCLC cells were treated with varying concentrations of both drugs for 72 hours with cell proliferation assessed using a cell viability assay. Interestingly, we observed synergistic anti-proliferative effects of DCC-3116 plus sotorasib in NCI-H2122 and Calu-1 cell lines (Figs. 1G-H), but not in NCI-H358 cells, which are noted to be exquisitely sensitive to single agent sotorasib (Fig. 1I). Using the Incucyte platform, we measured cell proliferation over time and noted that the combination of sotorasib plus DCC-3116 was superior to either single agent in NCI-H2122 and Calu-1 cells (Figs. 1D-E, Figs. S2D-E), but not in NCI-H358 cells (Fig, 1F, Fig. S2F). Intriguingly, we observed consistent inhibition of NCI-H358 cell proliferation with single agent DCC-3116 (Fig. 1F, Fig. S2F).
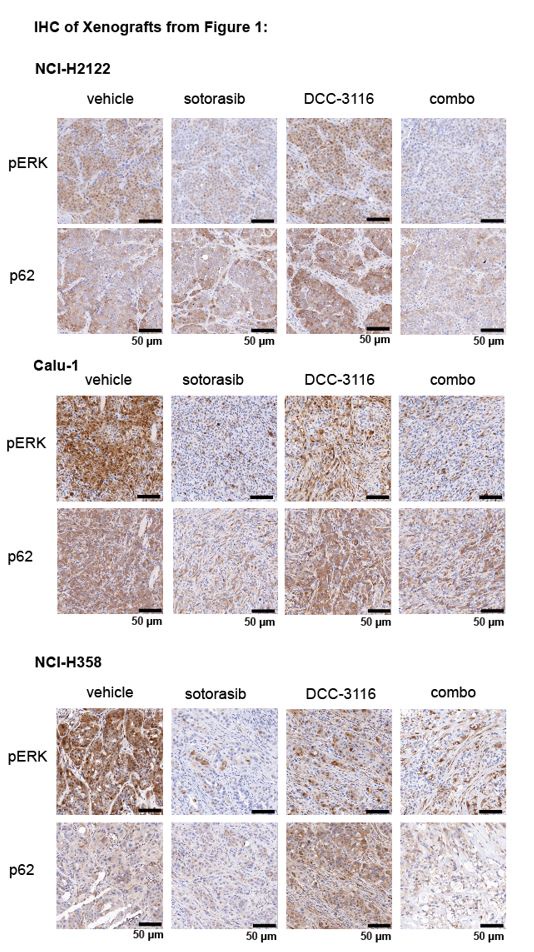
We next proceeded to test the anti-tumor effects of DCC-3116, either alone or in combination with sostorasib, against xenografted tumors derived from injection of NCI-H2122, Calu-1 or NCI-H358 cells into immunocompromised mice. Here, we observed clear cooperation of sotorasib plus DCC-3116 in tumors derived from NCI-H2122 and Calu-1 cells (Figs. 1J-K) but not from NCI-H358 cells, which are noted for their sensitivity to low-concentrations of sotorasib (Fig. 1L). To confirm the inhibitory activity of sotorasib in the cell lines used, cell extracts of control vs. sotorasib treated NSCLC cells were immunoblotted for phospho-ERK1/2. As expected, pERK1/2 was decreased in all cell lines following 2 hours of sotorasib treatment (Fig. S2G-I). In addition, we detected by immunoblotting the expected electrophoretic mobility shift of KRASG12C, which is indicative of covalent binding of sotorasib (561 daltons) to cysteine 12 of KRASG12C (Figs. S2G-I).27
Genetic inhibition of ULK1 decreases autophagy and cooperates with sotorasib to reduce cell viability
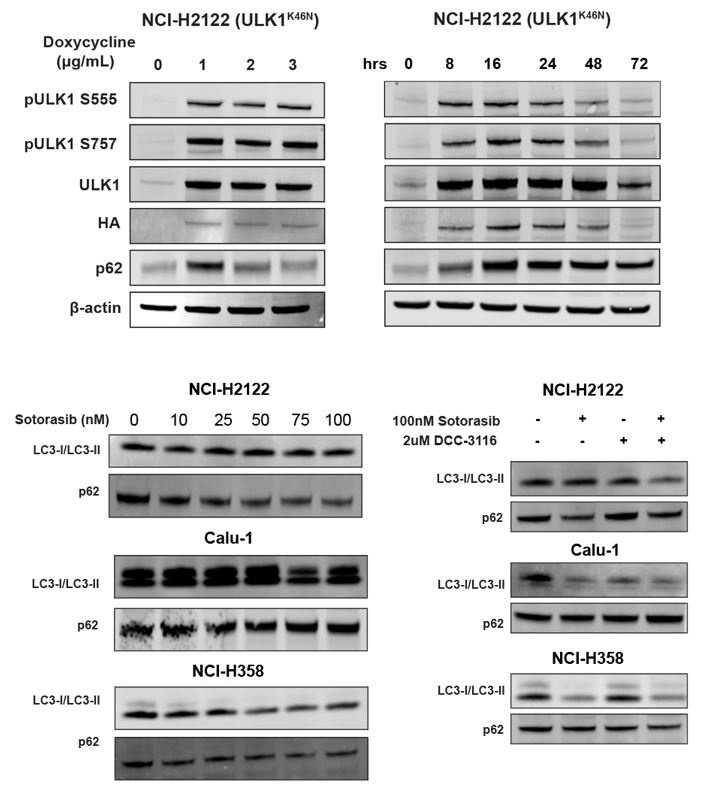
As an alternate way to assess the role of ULK1/2 in sotorasib-induced autophagy, we conditionally expressed a kinase-inactive, dominant negative form of ULK1 (ULK1K46N) under the control of a tetracycline transactivator (TetON)36. Doxycycline treatment of NCI-H2122/TetON::ULK1K46N cells led to a substantial increase in total ULK1 expression within 8 hours, which could be inferred to also be phosphorylated at known sites (pS555 and pS747) of regulatory phosphorylation of ULK1 (Figs. 2A-B). As anticipated, expression of ULK1K46N led to decreased phosphorylation of pS318-ATG13 in a manner comparable to treatment with DCC-3116 (Fig. 2C). In addition, expression of ULK1K46N led to a decrease in both basal and sotorasib-induced autophagy as assessed using the FAR. Finally, expression of ULK1K46N displayed synergistic anti-proliferative effects in NCI-H2122 cells when combined with sotorasib for 48 hours, suggesting that both genetic and pharmacological inhibition of ULK1 signaling had similar effects on NCI-H2122 cells (Fig. 2E).

Genetic inhibition of ULK1 decreases autophagy and cooperates with sotorasib to reduce cell viability.
A, Immunoblot of NCI-H2122:ULK1K46N cells after 24 hours of doxycycline treatment.
B, Immunoblot of NCI-H2122:ULK1K46N cells treated with 1 ug/mL doxycycline over time (hours).
C, ELISA assay of pS318-ATG13 expression after 16 hours of doxycycline treatment. Statistical significance was determined by an ordinary one-way ANOVA. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=3.
D, NCI-H2122:ULK1K46N cells were engineered to express the mCherry-EGFP-LC3 reporter, and a decrease in autophagy was demonstrated after 48 hours of doxycycline treatment. N=3. All statistical significance was measured using an ordinary one-way ANOVA with Dunnett’s multiple comparisons test. *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001
E, In vitro synergy assay of NCI-H2122:ULK1K46N cells treated with DMSO control, sotorasib and/or doxycycline over 48 hours using the Loewe method. N=3.
Either LKB1 silencing or expression of dominant-negative TP53R172H cooperates with KRASG12C-in GEM models of lung cancer
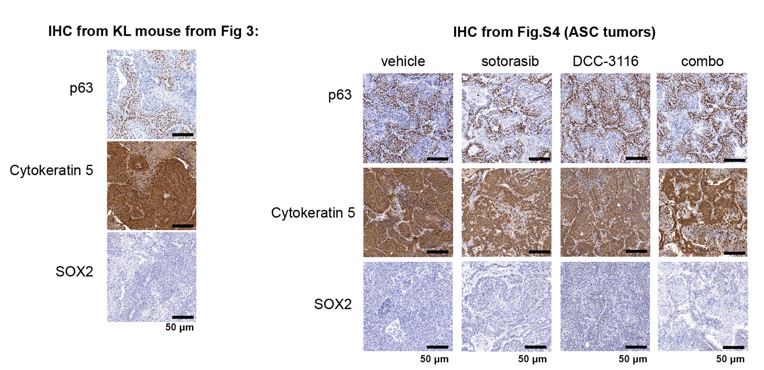
To complement the use of cultured human lung cancer cells, and to further test how additional genetic alterations influence KRASG12C-driven lung tumor progression and treatment response, we generated genetically engineered mouse (GEM) models of KRASG12C-driven lung cancer. To that end, we crossed KrasLSL-G12C/+ (K)24 mice to either: 1. Stk11fl/fl 37mice to generate KL mice or; 2. Trp53wm-R172H to generate KP mice38. Intranasal administration of an adenovirus encoding CRE recombinase under the control of a CMV promoter (Ad-CMV-CRE) to KL mice initiates expression of KRASG12C plus silencing of LKB1 in alveolar type 2 (AT2) cells. Similar treatment of KP mice initiates expression of KRASG12C plus dominant-negative TP53R172H in AT2 cells (Fig. 3A, Fig. S3A, Table 1). At euthanasia, 14 weeks post-initiation (p.i.), compared to KrasLSL-G12C/+ (K) mice, KL and KP mice displayed both accelerated lung cancer formation (Fig. 3B) and increased tumor burden (Fig. 3C). Histopathological analysis of H&E stained sections of KRASG12C only-driven lung tumors, also at 14 weeks p.i., indicated that they comprised mainly of atypical adenomatous hyperplasia (AAH) and low-grade small lung adenocarcinomas. KRASG12C/LKB1Null-driven lung tumors comprised AHH, adenocarcinomas, adenosquamous carcinomas (ASC) and mucinous adenocarcinomas. Finally, KRASG12C/TP53R172H-driven lung tumors consisted of AAH and a mix of low- and higher-grade lung adenocarcinomas (Fig. S3B). Immunohistochemical analysis of tumor sections indicated that phospho-ERK1/2 (pERK1/2) was detected in KRASG12C-driven lung tumors regardless of cooperating alterations in LKB1 or TP53 (Fig. 3D). However, the intensity of pERK1/2 staining across all three GEM models, and even between lesions in the same lung lobe (Fig. S3C) displayed substantial variability. Moreover, phospho-AKT1-3 (a surrogate for activation of PI3’-kinase signaling) was low across all KRASG12C-driven lung tumors, albeit that some individual cells in tumors had readily detected pAKT1-3 (Fig. S3D). Similar analysis confirmed the absence of LKB1 in KRASG12C/LKB1Null-driven lung tumors, but its presence in KRASG12C only and KRASG12C/TP53R172H-driven lung tumors. Similarly, KRASG12C/TP53R172H-driven lung tumors displayed elevated expression of TP53 that was not observed in KRASG12C only or in KRASG12C/LKB1Null-driven lung tumors (Fig. 3D). Previously published data demonstrated that KRAS-driven lung cancer cells occasionally suppress expression of NK2 homeobox 2 (NKX2.1), a master-regulatory transcription factor required for normal lung development39. In addition, KRAS-driven but NKX2.1 deficient lung tumors display a mucinous adenocarcinoma pathology and other transcriptional features of a pulmonary-to-gastric lineage switch 40. Although mucinous adenocarcinomas were detected in lung sections from KL mice (Fig. 3D), these KRASG12C/LKB1Null tumor cells retained expression of NKX2.1 (Fig. 3D, Fig. S3E), and some cells also expressed the gastrointestinal transcription factor HNF4α (Fig. S3E). Finally, we assessed expression of the AT2 specific pro-surfactant protein C (SPC), a surfactant important for the function of normal lung41, and noted its uniform expression in KRASG12C only driven lung tumors, but more variable expression in KRASG12C/LKB1Null- and KRASG12C/TP53R172H-driven lung tumors (Fig. 3D).

Either LKB1 silencing or expression of dominant-negative TP53R172H cooperates with KRASG12C in GEM models of lung cancer.
A, Schematic of genotypes of GEM models and abbreviations.
B, Representative images of lung lobes from GEM models at indicated time points post-initiation of lung tumorigenesis.
C, Quantification of lung tumor burdens from GEM models 14 weeks post-initiation of tumorigenesis. Statistical analysis was performed using an ordinary one-way ANOVA. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=4.
D, Hematoxylin and eosin (H&E) and immunohistochemical analysis of representative lung sections from GEM models 14 weeks post-initiation of tumorigenesis. p.i.=post initiation.
Any parts of this image created with BioRender are not made available under the same license as the Reviewed Preprint, and are © 2024, BioRender Inc.



Reagent Table
Anti-tumor effects of DCC-3116 and sotorasib either alone or in combination in GEM models of KRASG12C-driven lung cancer
The importance of autophagy in the initiation and/or progression of KRAS- or BRAF-driven lung cancer was revealed through analysis of GEM models in which deletion of key autophagy genes (Atg5 or Atg7) accompanied the initiation of oncoprotein expression42–46. Hence, we tested if DCC-3116 mediated inhibition of ULK1/2, starting at tumor initiation in KL mice, would inhibit tumor growth. Therefore, KL mice were initiated with Ad-CMV-CRE and then, starting 2 days p.i., treated with either vehicle or DCC-3116 (using a chow formulation) for 12 weeks (Fig. 4A) at which time mice were euthanized for analysis. DCC-3116 treated KL mice displayed significantly decreased tumor burden compared to control KL mice (Figs. 4B-C).

Combined inhibition of KRASG12C and ULK1/2 decreases tumor initiation and increases survival in KL GEMMs.
A, Schematic of lung tumor prevention dosing strategy of KL GEMMs. DCC-3116 was administered in drug-formulated chow. N=4
B, Representative images of lung lobes from GEM models 12 weeks post-initiation of tumorigenesis and DCC-3116 treatment.
C, Quantification of tumor burden of (B). Statistical analysis was measured by an unpaired student’s t-test. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=4
D, Schematic of treating tumor-bearing KL mice with vehicle control, 30 mg/kg sotorasib, chow containing DCC-3116 or the combination. DCC-3116 was administered in drug-formulated chow (Table 1). Mice were treated daily for 56 days or until termination criteria were reached, whichever was reached first. N=4-6.
E, Kaplan-Meyer survival curve of survival on treatment of KL mice treated as indicated. Statistical analysis was performed using a Log-Rank test. **p<.01 N=4-6
F, Quantification of tumor burden of (E).
G, H&E analysis of representative lung sections from KL mice after treatment. AB/PAS= Alcian Blue Periodic Acid Schiff, for staining mucins.
H- I, Quantification of immunohistochemical staining of treated mice pERK (H) and pAKT (I) as described in the methods. Statistical analysis was measured with an ordinary one-way ANOVA *p<0.05 **p<0.01 ns= not significant. N= 4-5
Any parts of this image created with BioRender are not made available under the same license as the Reviewed Preprint, and are © 2024, BioRender Inc.
To test if combined inhibition of KRASG12C plus ULK1/2 would inhibit growth of established KRASG12C/LKB1Null lung tumors, tumor-bearing KL mice at 10 weeks p.i. were administered sotorasib or DCC-3116 either alone or in combination for 8 weeks or until the mice met pre-determined euthanasia endpoints (Fig. 4D). Compared to vehicle, all three treatments significantly increased the survival of tumor-bearing KL mice (Fig. 4E). Although there was a trend towards improved survival of KL mice treated with the combination of sotorasib plus DCC-3116, these differences were not statistically significant (Fig. 4E). We also utilized micro-computed tomography (microCT) to monitor the response of tumors47 that were large enough to be detected throughout drug treatment (Fig. S4A). As expected, lung tumors in vehicle treated mice rapidly increased in size (Fig. S4A). The growth of KRASG12C/LKB1Null tumors in mice treated with sotorasib, DCC-3116 or the combination was delayed compared to those in vehicle treated mice, but tumors ultimately progressed on treatment (Fig. S4). However, some lung tumors in mice treated with the sotorasib plus DCC-3116 drug combination did not appear to change their size over the course of treatment (Fig. S4A). Throughout the course of treatment, we observed substantial (≤20%) weight loss in four out of five sotorasib only treated mice and one combination treated mouse that reached our euthanasia criteria (Fig. S4B). Following euthanasia, we performed immunohistochemical analysis on lung sections from control vs drug treated mice. In KL mice treated with sotorasib, either alone or in combination with DCC-3116, we noted a predominance of ASC and mucinous adenocarcinomas at the expense of AAH and adenocarcinoma (Fig. S4C). These results suggest that KRASG12C/LKB1Null-driven ASC or mucinous adenocarcinomas may be less sensitive to sotorasib treatment (Figs. S4A,C). Intriguingly, single-agent DCC-3116 treatment also slowed the growth of established tumors compared to vehicle treatment (Fig. S4A), but this did not inhibit the growth of AAH or adenocarcinomas (Fig. S4C). Immunohistochemistry revealed the expected decrease in pERK1/2 and pAKT1-3 staining in tumors treated with sotorasib, either alone or in combination with DCC-3116, compared to mice treated with vehicle or single-agent DCC-3116 alone (Figs. 4G-I). NKX2.1 expression was detected in all lung tumors regardless of the treatment group (Fig. 4G). Finally, many mice, regardless of the treatment type, developed lung tumors with the aforementioned mucinous adenocarcinoma phenotype, expressing HNF4αand stained postitive for various mucins (Alcian-Blue/PAS-positive) (Fig. 4G).
To further compare and contrast the effects of sotorasib and DCC-3116, either alone or in combination in GEM models of lung cancer and because of noted correlations between loss of LKB1 and insensitivity of cancers to various types of therapy, we employed our KP mouse model. 12,48 KRASG12C/TP53R172H- driven lung tumorigenesis was initiated in adult KP mice and at 10 weeks p.i. tumor bearing mice were treated with: 1. Vehicle control; 2. Sotorasib; 3. DCC-3116 or; 4. Combination of sotorasib plus DCC-3116 for 4 weeks (Fig. 5A) at which time mice were euthanized for analysis as described above. Interestingly, compared to control, there was a significant reduction in lung tumor burden in response to all three treatments (Fig. 5B). In addition, in all sotorasib treated mice, either alone or in combination with DCC-3116, regression of pre-existing lung tumors was detected by microCT scanning (Fig. 5C, Fig. S5). In mice treated with single agent DCC-3116, although an overall reduction in tumor burden was observed, some tumors detectable by microCT scanning continued to increase in size throughout treatment (Fig. 5B, Fig. S5). Moreover, in this experiment, we noted no weight loss in mice in any of the three treatment groups (Fig. 5C). Immunohistochemical analysis of lung sections from mice again revealed decreased pERK1/2 in mice treated with sotorasib either alone or in combination with DCC-3116, although pERK1/2 was detected in some areas of the lung (Figs. 5D-E). Vehicle treated KRASG12C/TP53R172H-driven lung tumors had detectable pAKT, which was decreased by all three treatments (Figs. 5D, F). Finally, all tumors displayed expression of NKX2.1 that was not substantially altered by drug treatment (Fig. 5D).

Inhibition of KRASG12C and ULK1/2 reduces tumor burden in a KP GEM model
A, Schematic of the treatment of KP GEM models. Mice were administered vehicle control or 30 mg/kg sotorasib once daily via oral gavage. DCC-3116 was administered in drug-formulated chow. N=4-5
B, Quantification of tumor burden of mice after 4 weeks of treatment. Statistical analysis was performed using an ordinary one-way ANOVA *p<0.05 ns=not significant. N=4-5
C, Percent change in the body weight of mice on treatment over 4 weeks. Each line depicts an individual mouse.
D, Representative images of histological analysis of lung lobes from KP mice 4 weeks after treatment.
E-F, Quantification of immunohistochemical staining of treated mice pERK1/2 (E) and pAKT1-3 (F) as described in the methods. Statistical analysis was performed using an ordinary one-way ANOVA *p<0.05 **p<0.01 ns= not significant
Any parts of this image created with BioRender are not made available under the same license as the Reviewed Preprint, and are © 2024, BioRender Inc.
KL lung-cancer-derived cells that acquire resistance to sotorasib increase RAS and pERK1/2 expression and do not increase autophagy after sotorasib treatment
To complement our in vivo work with GEM models and to better understand how acquired sotorasib resistance influences autophagy and treatment sensitivity, we generated KRASG12C/LKB1Null-lung cancer-derived cell lines from suitably manipulated, drug naive KL mice. As expected, parental KL70 cells are sensitive to the anti-proliferative effects of both sotorasib and the MEK1/2 inhibitor, trametinib (Figs. 6A-D). In addition, pERK1/2 also is inhibited by both sotorasib and trametinib (Fig. 6A). Next, we generated a sotorasib-resistant (SR) KRASG12C/LKB1Null-driven lung cancer cell line by culturing parental KL70 cells over 12 weeks in gradually increasing concentrations of sotorasib until a resistant population emerged (KL70SR cells). KL70SR cells were entirely resistant to the anti-proliferative effects of sotorasib but remained sensitive to trametinib (Fig. D). Consistent with this, the level of pERK1/2 was sotorasib resistant but trametinib sensitive (Fig. 6A). Indeed, KL70SR cells had a higher baseline level of both RAS and pERK1/2 expression compared to parental KL70 cells, which might explain their more rapid baseline proliferation (Fig. 6D).

KL lung-cancer-derived cells that acquire resistance to sotorasib increase RAS and pERK1/2 expression and do not increase autophagy after sotorasib treatment.
A, Immunoblot analysis of KL.70 and KL.70R cells treated with 100nM sotorasib or 100nM trametinib after 48 hours of treatment.
B-C, Quantification of signal from A normalized to b-actin.
D, Live cell imaging of percent confluence of KL.70 cells over time treated with DMSO, 100nm sotorasib of 100nM trametinib.
E, Autophagy measurement with FAR reporter in cells assessed by mCherry-eGFP-LC3 reporter after 48 hours of 100nM sotorasib or 100nM trametinib treatment. Red=high autophagy, Yellow= medium autophagy, Green= low autophagy. Statistical significance was determined by comparing autophagic flux levels to DMSO control and an ordinary one-way ANOVA with Dunnett’s multiple comparisons was used. Ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=9
F-G, In vitro synergy assay of KL70 cells treated with indicated doses of sotorasib and/or DCC-3116 using the Loewe method after 72 hours of treatment. N=3
H-I, In vitro synergy assay of KL70SR cells treated with indicated doses of sotorasib and/or DCC-3116 using the Loewe method after 72 hours of treatment. N=3
We next tested differences in baseline and drug-induced autophagy in KL70 and KL70SR cells using the fluorescent autophagy reporter. As expected based on analysis of human KRASG12C-driven lung cancer cells, KL70 cells increased autophagy in response to either sotorasib or trametinib treatment, a further demonstration that LKB1 is not required for increased autophagy in response to inhibition of KRASG12C>RAF>MEK>ERK inhibition (Fig. 6E). KL70SR cells treated with sotorasib displayed little induction of autophagy (Fig. 6E). However, trametinib elicited a robust induction of autophagy in KL70SR cells (Fig. 6E), further suggesting that changes in autophagy levels are correlated to pERK1/2 levels in the cell. Because sotorasib did not induce autophagy in the KL70SR cells, it was not clear if the addition of an autophagy inhibitor would decrease cell viability in the sotorasib-resistant cells. Cell viability was measured after 72 hours of drug treatment in the KL70 and KL70SR cell lines (Fig. 6 F-I). The combination of sotorasib and DCC-3116 did not lead to decreases in cell viability in the KL70SR cells (Fig. 6 H). We next tested if there were any synergist decreases in cell viability with the combination of trametinib and DCC-3116 because there was a robust increase in autophagy levels with trametinib treatment in the KL70SR cells. KL70SR cells are exquisitely sensitive to trametinib at very low concentrations and therefor little synergy was observed with the tested concentrations (Fig. I).
Discussion
Cancer cells exhibit increased autophagy in response to a variety of cellular stresses including nutrient deprivation or the application of pathway-targeted therapy, thereby allowing cancer cells to recycle a wide variety of macromolecules and organelles to promote cell viability.8,11 Moreover, GEM models of lung or pancreatic cancer indicate that cancer cells employ autophagy - both cancer cell autonomous and from the mouse as a whole - at a several stages of cancer progression46,49. Recently, we and others have demonstrated that many RAS-driven cancer cells display increased autophagy in response to blockade of RAS>RAF>MEK>ERK signaling 8,9,11. Moreover, in our work, combined inhibition of MEK1/2 (with trametinib) plus inhibition of lysosome function (with CQ or HCQ) displayed synergistic anti-proliferative activity in vitro, superior tumor control (compared to the single agents or standard of care) in cell line- or patient-derived xenografts (CDX or PDX) models of pancreatic cancer and, in one patient with advanced pancreatic cancer, there was evidence of anti-tumor activity of the combination of trametinib plus hydroxychloroquine8. These data (and others) have led to a number of clinical trials, some of which are ongoing (clinicaltrials.gov: NCT04214418, NCT04132505, NCT04386057, NCT04145297). Here we expand these observations using preclinical models of KRASG12C-driven lung cancer. The management of this disease has been transformed by FDA approval of adagrasib and sotorasib, covalent inhibitors of the KRASG12C oncoprotein27–29. However, as is generally true for single-agent pathway-targeted cancer therapy, the emergence of drug resistant disease can be rapid and pleiotropic50. Our data suggest that combined inhibition of KRASG12C plus autophagy may have superior activity against KRASG12C-driven lung cancer. These data are in accord with a recent study in which HCQ-mediated inhibition of lysosome function sensitized a KRASG12D/LKB1Null-driven GEM model of lung cancer to MEK1/2 inhibition 11. An interesting aspect of these results is the apparent dispensability of LKB1 for the induction of autophagy in response to inhibition of KRAS signaling. Our work and that of others had concluded that LKB1 was important for trametinib-induced autophagy in pancreatic cancer cells, but this new data argues that LKB1 is not an obligate requirement for autophagy induction8. Co-alterations in KRAS plus LKB1 are frequent in lung cancer and patients with these alterations are reported to display resistance to current therapeutic options such as chemotherapy and/or immunotherapy13,51,52. In addition, and perhaps surprisingly, we noted that DCC-3116 had substantial single-agent activity against KRASG12C-driven lung cancers in GEM models. Importantly, previous studies have demonstrated that combined inhibition of MEK1/2 plus autophagy does not lead to synergistic or cooperative decreases in cell proliferation or tumor growth in a GEM model of KRASG12D/TP53Null-driven lung cancer in which LKB1 expression is retained11. Here, we show that combined inhibition of KRASG12C plus ULK1/2 in Calu-1 cells (KRASG12C/LKB1WT/TP53null) decreased cell proliferation and tumor growth and that this combination was more effective than either single agent. Our findings demonstrate that inhibition of both KRASG12C plus ULK1/2 kinases is synergistic in KRASG12C mutant lung cancer cells with wild-type LKB1 expression but loss of TP53 expression. The difference between these findings could be due to the difference in KRAS missense mutation driving lung tumorigenesis or differences between inhibiting autophagy with a 4-aminoquiniolone such hydroxychloroquine and DCC-3116 mediated inhibition of ULK1/2. Indeed, since HCQ inhibits lysosome function, it might inhibit cellular recycling of substrates acquired by micropinocytosis, whereas ULK1/2 inhibition should be specific for autophagic recycling of intracellular substrates. Hence, this work supports the hypothesis that patients with KRASG12C-driven lung cancers may benefit from treatment with the combination of KRASG12C plus ULK1/2 inhibitors. To that end, there is an ongoing clinical trial enrolling patients with KRASG12C-driven cancers to test the combination of sotorasib plus DCC-3116 (NCT04892017).
Autophagy is reported to promote lung tumor progression by supporting tumor energy production43. Others have demonstrated that silencing of Autophagy-Related 7 (ATG7) expression, a protein essential for autophagy, in KRASG12D/LKB1null driven tumors at the time of tumor initiation substantially diminishes lung tumor burden43. Our study suggests that pharmacological inhibition of ULK1/2 kinase activity starting at tumor initiation also significantly reduces growth of KRASG12C/LKB1null lung tumors. Together, these data suggest that both ATG7 and ULK1/2 kinases are necessary for KRAS-driven lung tumorigenesis even when LKB1 expression is silenced. Intriguingly, treatment of mice bearing established KRASG12C-driven lung tumors with single agent DCC-3116 decreased tumor burden, further demonstrating that ULK-mediated autophagy may be important for maintenance of KRASG12C-driven lung tumors. Although we and others have demonstrated that autophagy inhibition as a monotherapy modestly reduces tumor burden in some preclinical models11,43, this has not been recapitulated in human cell line-derived models or in clinical trials with HCQ. This could be attributed to the relatively low potency of autophagy inhibition with HCQ, the low mutational burden of tumors arising in GEM models compared to human cancers or the use of non-specific autophagy inhibitors53–55. Indeed, we did observe that DCC-3116 treatment modestly inhibited the growth of one human KRASG12C mutant lung cancer cell line, NCI-H358, both in vitro and in vivo, suggesting that ULK1/2 inhibition as a monotherapy may indeed have significant single agent activity in certain settings.
In addition to assessment of preclinical therapeutic responses in GEM models, this study also addressed the histopathology of KRASG12C-driven lung tumors, either in combination with LKB1 silencing or expression of dominant-negative TP53R172H, in GEM models. In both the KRASG12C/LKB1Null and KRASG12C/TP53R172H GEM models of lung tumorigenesis, we noted the outgrowth of atypical adenomatous hyperplasia (AAH) and adenocarcinomas as was reported for KRASG12D-driven lung tumors some years ago. Interestingly, KRASG12C/LKB1Null-driven lung tumors also displayed mixed adenosquamous carcinomas (ASC) and mucinous adenocarcinomas that were not observed in the KRASG12C/TP53R172H-driven model. Analysis of the mucinous adenocarcinomas indicated that some tumor cells expressed the gastric lineage marker HNF4α, an observation originally reported in KRASG12D/NKX2.1Null-driven lung tumors40,56. In this (and related) GEM model(s), silencing of NKX2.1, a master transcriptional regulator of both lung and thyroid development, promoted the transition of lung tumor cells to a mucinous gastric cell phenotype through the action of HNF4α in partnership with the FOXA1/2 transcription factors56. Interestingly, HNF4α expressing KRASG12C/LKB1Null-driven lung tumor cells retained expression of NKX2.1 suggesting that NKX2.1 silencing is not required for the emergence of mucinous adenocarcinomas when LKB1 expression is silenced. Whether this reflects the ability of LKB1 signaling to: 1. Regulate cancer metabolism through effects on triosephosphate isomerase (TPI) or altered lactate production and secretion; 2. Regulate transcription effects on histone acetylation via elevated CRTC2-CREB signaling downstream of the salt-inducible kinases (SIKs) or effects on HDAC1/3 or some other signaling circuit remains to be determined57–62.
Finally, in both GEM models of KRASG12C-driven lung tumorigenesis, we observed inhibition of both AAH and adenocarcinoma with either single agent treatment or the combination of sotorasib plus DCC-3116. By contrast, in the GEM model of KRASG12C/LKB1Null-driven lung tumorigenesis, we noted an enrichment of the mixed adenosquamous and mucinous carcinomas after cessation of therapy. This suggests that this histopathological class of KRASG12C/LKB1Null-driven lung tumors may be relatively resistant to agents such as sotorasib that covalently inhibit the inactive, GDP-bound state of KRASG12C. This could reflect: 1. Differences in expression of RGS GTPase-activating proteins (GAPs) that promote GTP hydrolysis by KRASG12C; A cell state transition that is less dependent on the KRASG12C oncoprotein as has been described for resistance of EGFR-driven lung cancers to pathway-targeted inhibition of EGFR tyrosine kinase activity or; 3. Elevated expression of downstream factors such as c-MYC that we have recently shown can confer resistance to the combination of trametinib plus HCQ in models of KRAS-driven pancreatic cancer6,63,64.
In closing, our data support the testing of ULK1/2 inhibitors in patients with KRAS-driven lung cancer, either as single agents or in combination with direct inhibitors of KRAS oncoproteins, or of KRAS-regulated signaling pathways downstream of KRAS such as the RAF>MEK>ERK MAP kinase pathway. It will be interesting to interrogate specimens from patients on such trials prior to and after the likely eventual emergence of drug resistance to determine the extent to which potential resistance mechanisms glimpsed in preclinical models are observed in clinical trials. Such observations may pave the way for more efficacious regimens of therapy that maximize the depth and durability of desirable anti-tumor effects while minimizing toxicity.
Materials and methods
Cell Culture
Cell lines were routinely tested for mycoplasma contamination. All human lung cancer cell lines were obtained from the ATCC and cultured in RPMI (Roswell Park Memorial Institute) 1640 (Gibco 11875-093) supplemented with 10%(v/v) fetal bovine serum (FBS) (Gibco 10438-026) and 1% penicillin plus streptomycin (P/S) (Gibco 15140-122). Mouse tumor-derived cell lines were derived as previously described (PMID:19282848). Briefly, lungs from tumor-bearing mice were homogenized in a solution of digestive enzymes and plated in dishes. Cells were harshly split to allow for the outgrowth of tumor cells. The specific genetic abnormalities in each cell line were confirmed by analysis of genomic DNA or by immunoblotting. All mouse tumor-derived cell lines were cultured in DMEM/F12 (Dulbecco’s Modified Eagle Medium F12) (Gibco 11330-032) with 10%(v/v) FBS and 1% P/S.
Fluorescent Autophagy Reporter (FAR)
A vector encoding the chimeric FAR protein comprising mCherry:EGFP:LC3 was introduced into human and mouse cell lines as previously described with virus infected cells selected using puromycin and FAR expressing cells selected by flow cytometry.8,34 To assess autophagy, FAR expressing cells were seeded at equal densities in 6-well plates. After 24 hours, media was replaced with media containing the various drug treatments for 48 hours. Cells were harvested and stained with 1mM SYTOX Blue (Invitrogen S34857) for the exclusion of dead cells. Flow cytometry (Beckman Coulter CytoFLEX) was performed to assess mCherry and EGFP intensity and quantify the mCherry:EGFP ratio normalized to dimethyl sulfoxide (DMSO) control. Cells engineered to express the FAR are analyzed by flow cytometry in which we defined autophagy status by gating viable (based Sytox Blue staining), DMSO-treated control cells into three bins based on the ratio of EGFP:mCherry fluorescence. We gate all live cells into the 33% highest EGFP-positive cells (autophagy low) and the 33% highest mCherry-positive cells (autophagy high), and therefore, the proportion in the middle is also approximately 33% and considered the medium autophagy status. Again, these gates are based entirely on the DMSO-treated control cells, and all other treatments within the experiment are compared to settings on these gates. For statistical analysis, an ordinary one-way ANOVA and Dunnett’s multiple comparisons test were performed.
Lentiviral Transduction
HEK293T cells (Table 1) were seeded 24 hours prior to transfection in DMEM/F12 (10% FBS 1% P/S). DNA for lentivirus generation was introduced into these cells using a Lipofectamine 3000 kit (Invitrogen L3000015). All virus-containing supernatants were filtered through 0.45mm filters before use. To increase the efficiency of infection, 10mg/mL of Polybrene (MilliporeSigma TR-1003-G) was supplemented in the virus-containing media when added to cells. Cells were selected for successful infection through antibiotic selection with the corresponding antibiotics (puromycin or blasticidin) or flow cytometry for fluorescent markers. All plasmids used are described in Table 1 and are commercially available or previously published except the pLV-ULK1K46N plasmid.
ELISA assay for pS318-ATG13
pS318-ATG13 antibody (Rockland Immunochemicals, 600-401-C49) was biotinylated using a Biotin Labeling Kit-NH2 (Dojindo, LK03). ELISA plates (Corning, 9018) were coated in anti-ATG13 antibody (Table 1) overnight at 4 degrees. Cells were plated in equal densities in a 96-well plate. After 24 hours, cells were treated with various drug treatments for 16 hours, washed with 1x Phosphate-Buffered Saline (PBS), and then lysed in M-Per lysis buffer (Thermo Fisher, 78501) supplemented with 1x Ethylenediaminetetraacetic acid (EDTA, Thermo Fisher, 1861274), 1x Sigma Phosphatase Inhibitor (Sigma, 5726), 2x Halt Phosphatase and Protease inhibitor Cocktail (Thermo Fisher, 78446) for 15 minutes on an orbital shaker at 4°C. Cell extracts were then centrifuged for 10 minutes at 4,000 rpm in a 4°C refrigerated centrifuge. ELISA assay plates were washed twice with 1x ELISA wash buffer (Biolegend, 421601), blocked for 1 hour at RT with 1x ELISA dilutant buffer (Biolegend Cat# 421203), then washed twice with 1x wash buffer. Cell extracts were diluted in wash buffer, incubated in the ELISA plate at room temperature for 2 hours and then washed twice with 1x wash buffer. The biotinylated pS318-ATG13 antibody was diluted in 1x dilutant buffer and added to each well. After 1 hour at room temperature, wells were washed twice with 1x well wash buffer. Streptavidin-Poly-HRP antibody (Table 1) was diluted in 1x dilutant and added to each well for 1 hour at room temperature. Wells were washed twice with 1x well wash buffer and tetramethylbenzidine substrate (Biolegend, 421101) was added to each well for 15 minutes at room temperature. After 15 minutes, ELISA stop solution (Invitrogen, SS04) was added to each well. Background correction was performed by including control wells that received every reagent except for protein lysate and subtracting that value from all samples. Plates were immediately read on a Synergy HTX (BioTek) plate reader at 450nm and 540nm. Statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparisons.
Immunoblotting
Cell extracts were harvested as previously described7. Following clearing by centrifugation, protein lysates were quantified using a bicinchoninic acid assay (BCA) (Thermo Scientific 23250). 20-50mg samples of cell extract were fractionated by SDS-PAGE and then western blotted onto nitrocellulose membranes. Membranes were probed with primary antibodies (Table 1) overnight at 4°C. Secondary antibodies (Table 1) were diluted and incubated at room temperature for one hour. Membranes were imaged and analyzed on an Odyssey® CLx Infrared Imaging System (LI-COR®) and analyzed using Image Studio Software (LI-COR®).
In vitro synergy assays
Cells were seeded in triplicate at 5000-7000 cells/well into black-walled clear bottom 96-well plates (Costar 3603), cultured overnight in complete medium and then treated with various drug treatments at the indicated concentrations for 72 hrs. At the endpoint, ATP was quantified using CellTiter-Glo luminescent cell viability assay (Promega, G7570) per the manufacturer’s instructions. Luminescence was measured using a BioTek Synergy HTX plate reader and data were normalized to untreated controls. Synergy was determined using ComBenefit software (HSA, Bliss, Loewe models).65
Live Cell Imaging
Cells were imaged using an Incucyte Zoom Live Cell Imager (Sartorius) over time and analyzed using the IncuCyte Analysis Software (Sartorius). Cells were seeded at equal densities in 6-well plates and treated with various inhibitors either as single agents or in combination and imaged every two hours. Media was replaced after 48 hours for each well. Percent confluence and standard error was calculated at each time point. Statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparisons.
Generation of pLV-ULKK46N expression. Vector
The pLV-ULK1K46N plasmid was designed and constructed by VectorBuilder. 3x HA-tagged human ULK1K46N was cloned into a pLV lentivirus backbone vector containing a tetracycline-inducible promoter including a Tet response element and puromycin resistance cassette synthesized.
Animal Work
All animal work was approved by the Institutional Care and Use Committees at the University of Utah (protocol #: 21-1005). All work containing biohazardous agents was approved by the University of Utah Biosafety Committee. Mice were fed ad libitum, housed in micro isolator cages, and monitored daily in a temperature-controlled environment with a twelve-hour light/dark cycle. KrasLSL-G12C and Lkb1fl/fl mice were purchased from The Jackson Laboratory (Table 1)24,66. All mice were on a mixed genetic background. Both female and male mice were used for all experiments. Experimental adult mice were initiated between 6-8 weeks of age through nasal inhalation of adenovirus expressing CMV-Cre recombinase (Table 1) using 5x107 pfu per mouse as previously described67. Lung tumorigenesis was monitored in live mice with micro-computed tomography (microCT) scans (Perkin Elmer Quantum Gx) with a Cu 0.06+ AI 0.5 X-ray filter, 72 mm acquisition, and 45 mm reconstitution for a 2-minute standard scan. Mice were scanned up to 9 times (<1 gray of total radiation). Mice on treatment were weighed 2-3x per week and euthanized if unacceptable weight loss (>20%) was observed. Upon euthanasia, lungs were dissected and fixed using standard protocols68. For xenograft models, Nod.Cg-Prkdcscid/J mice were purchased from Jackson Laboratories (Table 1) or generated in-house. Cell lines were injected sub-cutaneously into the flank of adult mice at 1-5x106 cells per injection in a 1:1 mixture of serum-free Opti-MEM (Gibco, 31985-070) and Matrigel (Corning, 356231). Once tumors were palpable, they were measured using digital calipers. Once tumors reached ∼250mm3 mice were randomized to treatment arms. Mice were treated for 28 days or until maximum tumor volume was reached (∼1cm3). Tumor volume was calculated as equal to (tumor length) x (tumor width2)/2). Statistical analysis on xenograft assays was performed using an ordinary one-way ANOVA and unpaired student’s t-test. Corn oil vehicle (Sigma C8261) and sotorasib treatments were administered once daily via oral gavage. DCC-3116 was formulated in chow, and mice were fed ad libitum. Schematics illustrating treatment strategies were created using BioRender.
Survival of mice on treatment
KL mice were randomized to treatment arms at 10 weeks post tumor initiation (p.i.) and treated every day or until euthanasia end-point criteria were reached or after a pre-determined 56 days of drug treatment. Euthanasia criteria were defined per IACUC protocol to monitor pain and distress symptoms in mice69. Statistical analysis of survival was performed using the Log-rank (Mantel-Cox) test.
Tissue hematoxylin and eosin, immunohistochemistry staining
Lungs were harvested and inflated by perfusion through the trachea with 5 mLs of 10%(v/v) buffered formalin (Epredia 5725), and then fixed overnight with gentle rocking at room temperature following standard protocols68. Fixed lungs were embedded in paraffin and then 5 μm sections were prepared. Harris hematoxylin acidified (Epredia 6765003) and Eosin Y (Epredia 71211) stains were performed on all tissue sections. For immunohistochemistry (IHC), sections were first deparaffinized using standard protocols, and antigen retrieval was performed in a pressure cooker using 1x Citrate Buffer pH6 (Novus Biologicals, NB90062075). IHC was performed using the ImmPRESS® Polymer Reagents (Vector Laboratories). Primary antibodies (Table 1) were diluted and incubated at room temperature for 1 hour and then counter-stained with hematoxylin. Sections were dehydrated using standard protocols and mounted with ClearVue Mountant (Epredia 4212). Alcian Blue - Periodic Acid Schiff (PAS) staining was performed using standard protocols. Briefly, slides were deparaffinized, rehydrated and incubated in Alcian Blue (Leica 38016SS3A). Slides were washed and incubated in periodic acid (Leica 38016SS4A) followed by the Schiff Reagent (Fisher SS32-500).
Tissue Imaging and Quantification
All tissue imaging was performed using a 3D-Histech Midi Panoramic Scanner (Epredia) at 20x magnification. Tumor burden was quantified using CaseViewer software (Epredia). Total lung area and tumor area were digitally annotated using a closed polygon annotation. Total tumor and lung areas were summed, and the percent tumor burden was calculated. Immunohistochemical quantification was performed using 3D-Histech Quant Center software (Epredia) as previously described (Silvis et al. 2023). Statistical analysis was performed using an unpaired Student’s t-test.
Inhibitor Treatments
All pathway-targeted inhibitors (Table 1) were obtained from reputable sources and resuspended in the appropriate solvent (DMSO or water) for in vitro testing. The biochemical activity of all compounds was tested in vitro before being used in vivo. Compounds for in vivo dosing were resuspended in 10%(v/v) DMSO and 90%(v/v) corn oil (Table 1) or formulated in mouse chow. The DCC-3116 formulated mouse chow (Research Diets) was formulated with an OpenStandard Diet with 15% Kcal% Fat and 360 mg DCC-3116. CL/kg Diet.
Acknowledgements
We want to acknowledge the members of the McMahon and Kinsey labs for their valuable support, guidance, and sharing of reagents. P. Ghazi was supported by a JEDI award from the Life Sciences Editors Foundation, and we graciously thank Li-Kuo Su for editing suggestions and advice. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by the University of Utah Flow Cytometry Facility and the National Cancer Institute (NCI) through award number P30 CA042014. P. Ghazi would like to acknowledge funding provided by NCI under award number F31 CA261116. M. McMahon would like to acknowledge funding provided by NCI under award numbers R01 CA131261, R01 CA176839, and P30 CA042014, the Five for the Fight, the Huntsman Cancer Foundation, and the Huntsman Lung Cancer Disease Center. C. Kinsey would like to acknowledge funding provided by NCI award number K08 CA230151, the Huntsman Cancer Foundation, and the V Foundation Clinical Scholar Award. E. Snyder would like to acknowledge funding provided by the NCI under award numbers R01 CA212415, R01 CA240317 and R01 CA237404. G. Lozano would like to acknowledge funding provided by the NCI under award number R01 CA82577.
Disclosures: Research described here was supported through a Sponsored Research Agreement between the University of Utah and Deciphera Pharmaceuticals and awarded to M.M and C.G.K. M.M has previously but no longer been retained as a paid consultant to Deciphera Pharmaceuticals; C.G.K reported personal fees from Deciphera Pharmaceuticals outside the submitted work. M.B and D.L.F are stockholders of Deciphera Pharmaceuticals. No other disclosures were reported.

Human KRAS-driven lung cancer cells increase autophagy after KRASG12C or MEK inhibition.
A-E, Autophagy levels in human KRASG12C-driven lung cancer cells Calu-1 (A), NCI-H2122 (B), NCI-H23 (C), NCI-H358 (D) and the KRASG12V-driven human lung cancer cell line Cor-L23 (E) after 48 hours of sotorasib at indicated concentrations with the FAR reporter. Red=high autophagy, Yellow= medium autophagy, Green= low autophagy. Statistical significance was determined by comparing autophagic flux levels to DMSO control and an ordinary one-way ANOVA with Dunnett’s multiple comparisons was used. ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=9
F-J, Autophagy levels in human KRASG12C-driven lung cancer cells Calu-1 (F), NCI-H2122 (G), NCI-H23 (H), NCI-H358 (I) and the KRASG12V-driven human lung cancer cell line Cor-L23 (J) after 48 hours of trametinib at indicated concentrations with the FAR reporter. Red=high autophagy, Yellow= medium autophagy, Green= low autophagy. Statistical significance was determined by comparing autophagic flux levels to DMSO control and an ordinary one-way ANOVA with Dunnett’s multiple comparisons was used. ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=9

Human KRASG12C-driven lung cancer cell lines increase autophagy and decrease cellular proliferation after sotorasib treatment.
A-C, ELISA measurement of pS318-ATG13 signal after 16 hours of drug treatment in NCI-H2122 (A), Calu-1 (B) and NCI-H358 cell lines (C). N=3. Statistical analysis was performed using an ordinary one-way ANOVA with Tukey’s multiple comparisons test. ns= not significant, *p<0.05 ****p<0.0001.
D-F, Percent confluence over time of NCI-H2122 (D), Calu-1 (E) and NCI-H358 (F) cell lines treated with indicated compounds over time. Statistical analysis was performed using an ordinary one-way ANOVA with Tukey’s multiple comparisons test. ns= not significant, *p<0.05 **p<0.01 ***p<0.001 ****p<0.0001. N=3-5
G-I, Immunoblotting analysis of NCI-H2122 (G), Calu-1 (H), and NCI-H358 (I) cell lines after 100nM sotorasib treatment over 24 hours.

Loss of LKB1 expression leads to mixed ASC and mucinous ADC lung tumors in KRASG12C-driven GEMMs.
A, Schematic of allele, structure, and protein changes in GEM models used.
B, Representative hematoxylin and eosin stains of K, KL and KP GEM models 14 weeks post-initiation. N.O.= not observed, AAH= atypical adenomatous hyperplasia, ADC= adenocarcinoma, ASC= adenosquamous cell carcinoma, mucinous ADC= mucinous adenocarcinoma.
C-D, Representative immunohistochemical stains of pERK1/2 (C) and pAKT1-3 (D) signal in K, KL and KP GEM models.
E, Representative images of hematoxylin and eosin (H&E) staining and immunohistochemistry of indicated proteins in KL mice.

MicroCT, body weight and pathological analysis of treated KL mice.
A, Representative microCT images of KL mice pre-treatment (Day 0) and 14 days, 28 days and 56 days post-treatment. Vehicle-treated mice and most DCC-3116 treated mice reached termination criteria before the 56-day endpoint. Red arrows indicate lung tumors.
B, Percent change in the body weight of mice over the course of the treatment period. Each line depicts an individual mouse.
C, Representative images of hematoxylin and eosin staining of lung sections of KL mice at the end of treatment. N.O.= not observed, AH= alveolar hyperplasia, ADC= adenocarcinoma, ASC= adenosquamous cell carcinoma, mucinous ADC= mucinous adenocarcinoma.

MicroCT images of KP mice on treatment.
Representative microCT images of KP mice pre-treatment (Day 0) and 7 days, 14 days and 28 days post-treatment. Red arrows indicate lung tumors.
References
- 1.Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinomaClin Cancer Res 14:5731–4Google Scholar
- 2.Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lungCancer 92:1525–30Google Scholar
- 3.KRAS: From undruggable to a druggable Cancer TargetCancer Treat Rev 89:102070Google Scholar
- 4.K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactionsNature 503:548–51Google Scholar
- 5.Targeting KRAS Mutant Cancers with a Covalent G12C-Specific InhibitorCell 172:578–589Google Scholar
- 6.MYC-mediated resistance to trametinib and HCQ in PDAC is overcome by CDK4/6 and lysosomal inhibitionJ Exp Med 220Google Scholar
- 7.Chloroquine Sensitizes GNAQ/11-mutated Melanoma to MEK1/2 InhibitionClin Cancer Res 26:6374–6386Google Scholar
- 8.Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancersNat Med 25:620–627Google Scholar
- 9.Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancerNat Med 25:628–640Google Scholar
- 10.LKB1 and cancer: The dual role of metabolic regulationBiomed Pharmacother 132:110872Google Scholar
- 11.Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumorsCell Death Dis 14:61Google Scholar
- 12.LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenforminCancer Cell 23:143–58Google Scholar
- 13.The role of LKB1 in lung cancerFam Cancer 10:447–53Google Scholar
- 14.A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)Annals of Oncology 26:894–901Google Scholar
- 15.Autophagy Agents in Clinical Trials for Cancer Therapy: A Brief ReviewCurrent Oncology 29:1695–1708Google Scholar
- 16.Toll-like receptorsAnnu Rev Immunol 21:335–76Google Scholar
- 17.Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseasesJ Antimicrob Chemother 70:1608–21Google Scholar
- 18.Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptinEur J Biochem 95:215–25Google Scholar
- 19.Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusionAutophagy 14:1435–1455Google Scholar
- 20.Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous MechanismsCancer Discov 8:276–287Google Scholar
- 21.Autophagy: cellular and molecular mechanismsJ Pathol 221:3–12Google Scholar
- 22.The mammalian ULK1 complex and autophagy initiationEssays Biochem 61:585–596Google Scholar
- 23.Enhancing Anti-Cancer Therapy with Selective Autophagy Inhibitors by Targeting Protective AutophagyBiomol Ther (Seoul 31:1–15Google Scholar
- 24.An In Vivo Kras Allelic Series Reveals Distinct Phenotypes of Common Oncogenic VariantsCancer Discov 10:1654–1671Google Scholar
- 25.Clinical characteristics and prognosis of patients with lung adenosquamous carcinoma after surgical resection: results from two institutesJ Thorac Dis 10:2397–2402Google Scholar
- 26.Adenosquamous carcinoma of the lungOnco Targets Ther 11:4829–4835Google Scholar
- 27.The clinical KRAS(G12C) inhibitor AMG ti10 drives anti-tumour immunityNature 575:217–223Google Scholar
- 28.Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of CancerJ Med Chem 63:6679–6693Google Scholar
- 29.The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and PatientsCancer Discov 10:54–71Google Scholar
- 30.Acquired Resistance to KRASG12C Inhibition in CancerNew England Journal of Medicine 384:2382–2393Google Scholar
- 31.Diverse alterations associated with resistance to KRAS(G12C) inhibitionNature 599:679–683Google Scholar
- 32.Resistance to KRASG12C Inhibitors in Non-Small Cell Lung CancerFront Oncol 11:787585Google Scholar
- 33.Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK ReactivationCancer Discov 11:1913–1922Google Scholar
- 34.Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3Autophagy 3:452–60Google Scholar
- 35.Global profiling of AMG510 modified proteins identified tumor suppressor KEAP1 as an off-targetiScience 26:106080Google Scholar
- 36.FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cellsJ Cell Biol 181:497–510Google Scholar
- 37.Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cellsNature 468:653–8Google Scholar
- 38.Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndromeCell 119:861–72Google Scholar
- 39.NKX2-1 expression as a prognostic marker in early-stage non-small-cell lung cancerBMC Pulm Med 17:197Google Scholar
- 40.Nkx2-1 represses a latent gastric differentiation program in lung adenocarcinomaMol Cell 50:185–99Google Scholar
- 41.8 - Alveolar Epithelium and Pulmonary SurfactantIn: Murray and Nadel’s Textbook of Respiratory Medicine (Sixth EdiEon) Philadelphia: W.B. Saunders pp. 134–149https://doi.org/10.1016/B978-1-4557-3383-5.00008-7Google Scholar
- 42.Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasisGenes Dev 27:1447–1461Google Scholar
- 43.Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesisGenes Dev 33:150–165Google Scholar
- 44.Autophagy compensates for Lkb1 loss to maintain adult mice homeostasis and survivalElife 9Google Scholar
- 45.Autophagy maintains tumour growth through circulating arginineNature 563:569–573Google Scholar
- 46.A dual role for autophagy in a murine model of lung cancerNat Commun 5:3056Google Scholar
- 47.Imaging techniques for small animal imaging models of pulmonary disease: micro-CTToxicol Pathol 35:59–64Google Scholar
- 48.STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic ImpactCells 10Google Scholar
- 49.Regulation and function of autophagy in pancreatic cancerAutophagy 17:327–3296Google Scholar
- 50.Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome ResistanceCancers (Basel) 14Google Scholar
- 51.Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lungCancer Res 62:3659–62Google Scholar
- 52.Co-occurring KRAS mutation/LKB1 loss in non-small cell lung cancer cells results in enhanced metabolic activity susceptible to caloric restriction: an in vitro integrated multilevel approachJ Exp Clin Cancer Res 37:302Google Scholar
- 53.Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinomaOncologist 19:637–8Google Scholar
- 54.Targeting autophagy addiction in cancerOncotarget 2:1302–6Google Scholar
- 55.Targeting Autophagy in Cancer: Recent Advances and Future DirectionsCancer Discov 9:1167–1181Google Scholar
- 56.FoxA1 and FoxA2 drive gastric differentiation and suppress squamous identity in NKX2-1-negative lung cancerElife 7Google Scholar
- 57.LKB1-Dependent Regulation of TPI1 Creates a Divergent Metabolic Liability between Human and Mouse Lung AdenocarcinomaCancer Discov 13:1002–1025Google Scholar
- 58.HDAC3 is critical in tumor development and therapeutic resistance in Kras-mutant non-small cell lung cancerSci Adv 9:eadd3243Google Scholar
- 59.The AMPK-Related Kinases SIK1 and SIK3 Mediate Key Tumor-Suppressive Effects of LKB1 in NSCLCCancer Discov 9:1606–1627Google Scholar
- 60.The LKB1-AMPK pathway: metabolism and growth control in tumour suppressionNat Rev Cancer 9:563–75Google Scholar
- 61.MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinomaCancer Cell 41:1363–1380Google Scholar
- 62.LKB1 controls inflammatory potential through CRTC2-dependent histone acetylationMol Cell 83:1872–1886Google Scholar
- 63.The G protein signaling regulator RGS3 enhances the GTPase activity of KRASScience 374:197–201Google Scholar
- 64.Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitorsSci Transl Med 3:75ra26Google Scholar
- 65.Combenefit: an interactive plarorm for the analysis and visualization of drug combinationsBioinformatics 32:2866–8Google Scholar
- 66.Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cellsNature 468:653–8Google Scholar
- 67.Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivoJ Clin Invest 102:184–93Google Scholar
- 68.Instillation and Fixation Methods Useful in Mouse Lung Cancer ResearchJ Vis Exp :e52964https://doi.org/10.3791/52964Google Scholar
- 69.Body condition scoring: a rapid and accurate method for assessing health status in miceLab Anim Sci 49:319–23Google Scholar
Article and author information
Author information
Version history
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.96992. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Ghazi et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.