Author response:
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
In this work, the authors investigate the functional difference between the most commonly expressed form of PTH, and a novel point mutation in PTH identified in a patient with chronic hypocalcemia and hyperphosphatemia. The value of this mutant form of PTH as a potential anabolic agent for bone is investigated alongside PTH(1-84), which is a current anabolic therapy. The authors have achieved the aims of the study. Their conclusion that this suggests a "new path of therapeutic PTH analog development" seems unfounded; the benefit of this PTH variant is not clear, but the work is still interesting.
The work does not identify why the patient with this mutation has hypocalcemia and hyperphosphatemia; this was not the goal of the study, but the data is useful for helping to understand it.
Thank you for your valuable feedback. In this study, we confirmed that R25CPTH can form a dimer, and our in vivo experiments in the mouse model demonstrated that dimeric R25CPTH can stimulate bone formation similarly to normal PTH. Furthermore, patients with the R25CPTH mutation, who have been exposed to high levels of this variant over an extended period, were reported to have high bone mineral density. Based on these observations, we hypothesized that dimeric R25CPTH might have potential as a new therapeutic PTH analog, particularly as a bone anabolic agent. However, we acknowledge that it is premature to make definitive claims regarding its therapeutic utility. Thus, we are currently conducting follow-up research to further investigate the subsignaling pathway changes induced by dimeric R25CPTH and their impact on bone metabolism.
Moreover, to fully understand the patient’s symptoms, it is crucial to determine the form in which R25CPTH exists in vivo. While our in vitro experiments demonstrated that R25CPTH is secreted primarily in its dimeric form, we do not yet know whether this dimeric structure is maintained in vivo. We are actively conducting experiments to analyze the circulating form of R25CPTH in patients through blood sample collection (Andersen et al., 2022; Lee et al., 2015). Should the mutation predominantly exist in its monomeric form in vivo, this would align with clinical findings reported by Lee et al. (2015), which could help explain the patient’s hypocalcemia and hyperphosphatemia. However, if R25CPTH primarily exists in its dimeric form, additional research will be necessary to uncover the underlying mechanisms. Based on our experimental results, the dimeric R25CPTH exhibits a reduced binding affinity to PTH1R compared to the monomeric form. Furthermore, our in vitro experiments revealed that dimeric R25CPTH induces lower levels of cAMP production upon PTH1R activation. Accordingly, we can assume that this reduction in receptor signaling is likely to account for the impaired regulation of calcium and phosphate in patients with the mutation. However, despite this diminished signaling in calcium and phosphate homeostasis, dimeric R25CPTH was still capable of promoting bone formation at levels comparable to wild-type PTH. This apparent paradox warrants further investigation, and we are actively pursuing studies to elucidate how the dimeric form exerts its effects on bone metabolism.
References
Andersen, S. L., Frederiksen, A. L., Rasmussen, A. B., Madsen, M., & Christensen, A. R. (2022). Homozygous missense variant of PTH (c.166C>T, p.(Arg56Cys)) as the cause of familial isolated hypoparathyroidism in a three-year-old child. J Pediatr Endocrinol Metab, 35(5), 691-694. https://doi.org/10.1515/jpem-2021-0752
Lee, S., Mannstadt, M., Guo, J., Kim, S. M., Yi, H. S., Khatri, A., Dean, T., Okazaki, M., Gardella, T. J., & Juppner, H. (2015). A Homozygous [Cys25]PTH(1-84) Mutation That Impairs PTH/PTHrP Receptor Activation Defines a Novel Form of Hypoparathyroidism. J Bone Miner Res, 30(10), 1803-1813. https://doi.org/10.1002/jbmr.2532
Strengths:
The work is novel, as it describes the function of a novel, naturally occurring, variant of PTH in terms of its ability to dimerise, to lead to cAMP activation, to increase serum calcium, and its pharmacological action compared to normal PTH.
Weaknesses:
(1) The use of very young, 10 week old, mice as a model of postmenopausal osteoporosis remains a limitation of this study, but this is now quite clearly described as a limitation, including justifying the use of the primary spongiosa as a measurement site.
We appreciate the reviewer’s comment.
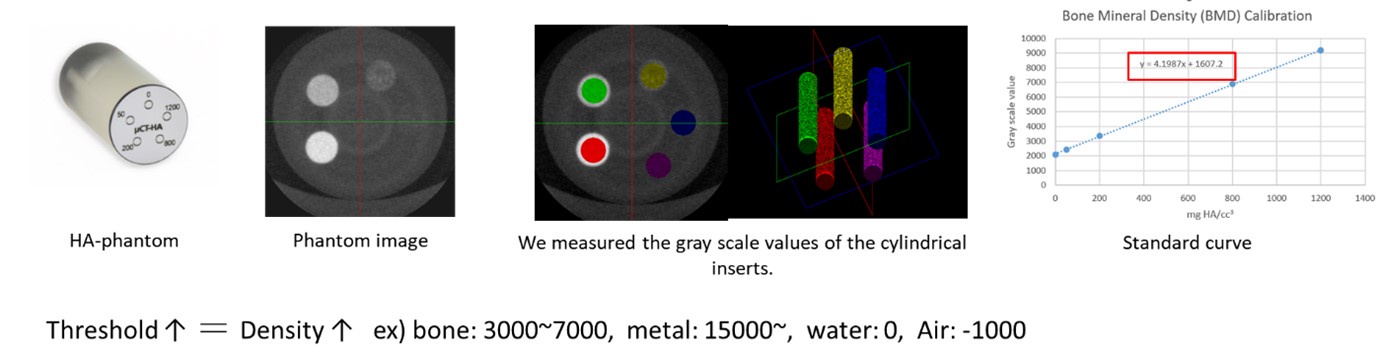
(2) Methods have been clarified. It is still necessary to properly define the micro-CT threshold in mm HA/cc^3. I think it might be acat about 200mg HA/cc^3 in this study.
Thank you for your insightful comment. To address this, we utilized hydroxyapatite (HA) phantom with HA content ranging from 0 to 1200 mg/cm3, with calibration points at 0, 50, 200, 800, 1000, and 1200 mg CaHA/cm3, to measure grayscale values via µ-CT. Based on these measurements, the trabecular bone BMD in our study was determined to range from 100 to 200 mg/cm3.
Author response image 1.
(3) The apparent contradiction between the cortical thickness data (where there is no difference between the two PTH formulations) and the mechanical testing data (where there is a difference) remains unresolved. It is still not clear whether there is a material defect in the bone, which can be partially assessed by reporting the 3-point bending test, corrected for the diameters of the bone (i.e. as stress / strain curves).
Thank you for your comment. First, we ensured that the bones sampled during the experiment showed no defects, and we carefully separated the femur bones from the mice to preserve their integrity. In the 3-point bending test, PTH treatment significantly increased the maximum load of the femur bone compared to the OVX-control group. Additionally, the maximum load in the PTH treatment group was significantly greater than that observed in the PTH dimer group. Furthermore, structural factors influencing bone strength, such as the perosteal perimeter and the endocortical bone perimeter, were also increased in the PTH treatment group compared to the PTH dimer group (data only for reviewer).
Author response image 2.
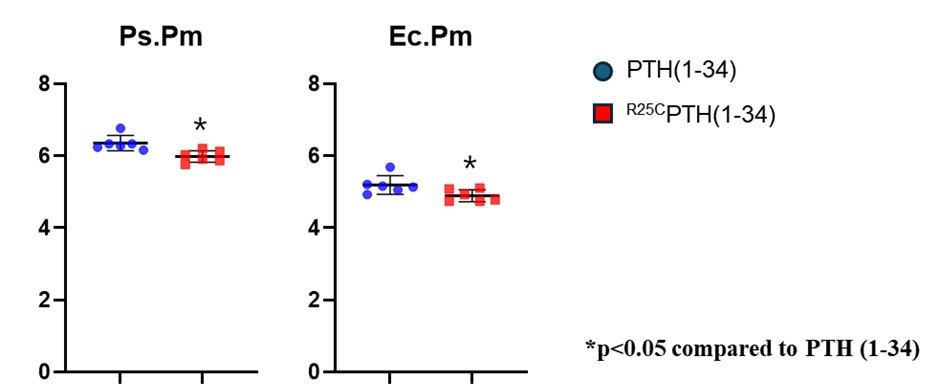
(4) It is also puzzling that both dimeric and monomeric PTH lead to a reduction in total bone area (cross sectional area?). This would suggest a reduction in bone growth. This should be discussed in the work.
In our experiment, the data showed an increase in cortical bone area in the PTH treatment group, but not in the PTH dimer treatment group. However, both dimeric and monomeric PTH treatments resulted in a reduction in total tissue area. We added revised sentence in page 13 line 317 and page 14 line 333 as follows:
“In addition, the data showed an increase in cortical bone area (Ct.Ar) in the PTH treatment group but not in the PTH dimer treatment group. However, both dimeric and monomeric PTH treatments reduced total tissue area (Tt.Ar), suggesting potential effects on bone growth in the width of mice or humans.”
“This study has several limitations. First, it is urgently necessary to determine whether dimeric R25CPTH is present in human patient serum. Second, TRAP staining showed an inhibitory effect of PTH treatment on the primary spongiosa area. However, the secondary spongiosa, which more accurately reflects bone remodeling (55), was not examined due to the barely detectable bone in this area in OVX-induced osteoporosis mouse models. Third, it is unclear whether similar bone phenotypes exist between human R25CPTH patients and dimeric R25CPTH-treated mice, particularly regarding low bone strength. Although the dimeric R25CPTH-treated group showed higher cortical BMD compared to WT-Sham or PTH groups, there was no difference in bone strength compared to the osteoporotic mouse model. Fourth, our study demonstrated that PTH or R25CPTH treatment decreased circumferential length, which could affect bone growth in width. However, whether this phenotype is also observed in patients treated with PTH or R25CPTH remains uncertain.”





