Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
The model of phosphotransfer from Y169 IKK to S32 IkBa is compelling and an important new contribution to the field. In fact, this model will not be without controversy, and publishing the work will catalyze follow-up studies for this kinase and others as well. As such, I am supportive of this paper, though I do also suggest some shortening and modification.
We appreciate the reviewers candid response on the difficulty of this study and the requirement of follow-up studies to confirm a direct transfer of the phosphate. We also have edited the manuscript to make it shorter.
Generally, the paper is well written, but several figures should be quantified, and experimental reproducibility is not always clear. The first 4 figures are slow-going and could be condensed to show the key points, so that the reader gets to Figures 6 and 7 which contain the "meat" of the paper.
We have indicated the experimental reproducibility in the methodology section against each assay. We have shortened the manuscript corresponding to sections describing figures 1-4. However, when we talked to some of our colleagues whose expertise do not align with kinases and IKK, we realized that some description were necessary to introduce them to the next figures. Additionally, we added Fig. S6 indicating that the radiolabelled phospho-IKK2 Y169F is unable to transfer its own phosphate group(s) to the substrate IkBa.
Reviewer #2 (Public Review):
Phosphorylation of IκBα is observed after ATP removal, although there are ambiguous requirements for ADP.
We agree with the reviewer that this observation is puzzling. We hypothesize that ADP is simultaneously regulating the transfer process likely through binding to the active site.
It seems that the analysis hinges on the fidelity of pan-specific phosphotyrosine antibodies.
We agree with the reviewer. To bolster our conclusion, we used antibodies from two different sources. These were Monoclonal mouse anti-Phospho-Tyrosine (catalogue number: 610000) was from BD Biosciences or from EMD Millipore (catalogue no. 05-321X).
The analysis often returns to the notion that tyrosine phosphorylation(s) (and critical active site Lys44) dictate IKK2 substrate specificity, but evidence for this seems diffuse and indirect. This is an especially difficult claim to make with in vitro assays, omitting the context of other cellular specificity determinants (e.g., localization, scaffolding, phosphatases).
We agree with the concerns that the specificity could be dependent on other cellular specificity determinants and toned down our claims where necessary. However, we would like to point out that the specificity of IKK2 towards S32 and S36 of IkBa in cells in response to specific stimuli is well-established. It is also well-established that its non-catalytic scaffolding partner NEMO is critical in selectively bringing IkBa to IKK from a large pool of proteins. The exact mechanism of how IKK2 choose the two serines amongst many others in the substrate is not clear.
Multiple phosphorylated tyrosines in IKK2 were apparently identified by mass spectrometric analyses, but the data and methods are not described. It is common to find non-physiological post-translational modifications in over-expressed proteins from recombinant sources. Are these IKK2 phosphotyrosines evident by MS in IKK2 immunoprecipitated from TNFa-stimulated cells? Identifying IKK2 phosphotyrosine sites from cells would be especially helpful in supporting the proposed model.
Mass spectrometric data for identification of phosphotyrosines from purified IKK2 is now incorporated (Figure S3A). Although we have not analyzed IKK2 from TNF-a treated cells in this study, a different study of phospho-status of cellular IKK2 indicated tyrosine phosphorylation (Meyer et al 2013).
Reviewer #3 (Public Review):
The identity and purity of the used proteins is not clear. Since the findings are so unexpected and potentially of wide-reaching interest - this is a weakness. Similar specific detection of phospho-Ser/Thr vs phospho-Tyr relies largely on antibodies which can have varying degrees of specificity.
We followed a stringent purification protocol of several steps (optimized for the successful crystallization of the IKK2) that removed most impurities (PMID: 23776406, PMID: 39227404). The samples analysed with ESI MS did not show any significant contaminating kinase from the Sf9 cells.
Sequence specific phospho-antibodies used in this study are very well characterized and have been used in the field for years (Basak et al 2007, PMID: 17254973). We agree on the reviewer’s concerns on the pan-specific phospho-antibodies. Since phospho-tyrosine detection is the crucial aspect of this study, we minimized such bias by using pan-specific phosphotyrosine antibodies from two independent sources.
Reviewer #1 (Recommendations For The Authors):
I understand that Figure 3 shows that K44M abolishes both S32/26 phosphorylation and tyrosine phosphorylation, but not PEST region phosphorylation. This suggests that autophosphorylation is reflective of its known specific biological role in signal transduction. But I do not understand why "these results strongly suggest that IKK2-autophosphorylation is critical for its substrate specificity". That statement would be supported by a mutant that no longer autophosphorylates, and as a result shows a loss of substrate specificity, i.e. phosphorylates non-specific residues more strongly. Is that the case? Maybe Darwech et al 2010 or Meyer et al 2013 showed this.
Later figures seem to address this point, so maybe this conclusion should be stated later in the paper.
We have now clarified this in the manuscript and moved the comment to the next section. We have consolidated the results in Figure 3 and 4 in the previous version into a single figure in Figure. The text has also been modified accordingly.
Page 10: mentions DFG+1 without a proper introduction. The Chen et al 2014 paper appears to inform the author's interest in Y169 phosphorylation, or is it just an additional interesting finding? Does this publication belong in the Introduction or the Discussion?
The position of Y169 at the DFG+1 was intriguing and the 2014 article Chen et al further bolstered our interest in this residue to be investigated. We think this publication is important in both sections.
To understand the significance of Figure 4D, we need a WT IKK2 control: or is there prior literature to cite? This is relevant to the conclusion that Y169 phosphorylation is particularly important for S32 phosphorylation.
We have now added a new supplementary figure where activities of WT and Y169F IKK2 towards WT and S32/S36 mutants are compared (Figure S3F). At a similar concentration, the activity of WT-IKK2 is many fold higher than that of YtoF mutants (Fig. 4C). The experiments were performed simultaneously, although samples were loaded on different gels but otherwise processed in a similar way. The corresponding data is now included in the manuscript as Figure S3F.
The cold ATP quenching experiment is nice for testing the model that Y169 functions as a phospho sink that allows for a transfer reaction. However, there is only a single timepoint and condition, which does not allow for a quantitative analysis. Furthermore, a positive control would make this experiment more compelling, and Y169F mutant should show that cold ATP quenching reduces the phosphorylation of IkBa.
We thank the reviewer for appreciating our experimental design, and pointing out the concerns. We kept the ATP-time point as the maximum of the non-competition experiment. Also, we took 50mM ATP to compare its competition with highest concentration of ADP used. The idea behind using the maximum time and ATP (comparable to ADP) was to capture the effect of competitive-effect of ATP, if any, that would be maximal in the given assay condition in comparison with the phospho-transfer set up in absence of cold ATP. We agree that finer ranges of ATP concentration and time points would have enabled more quantitative analyses. We have now included data where different time intervals are tested (Figure S5D).
Why is the EE mutant recognized by anti-phospho-serine antibodies? In Figure 2F.
We anticipate Serine residues besides those in the activation loop to be phosphorylated when IKK2 is overexpressed and purified from the Sf9 cells. Since Glu (E) mimics phospho-Ser, the said antibody cross reacts with the IKK2-EE that mimics IKK2 phosphorylated at Ser177 and 181.
Figure 7B is clear, but 7C does not add much.
We have now removed the Fig. 7C in the current version. Figure 7 is now renumbered as Figure 6 that does not contain the said cartoon.
Reviewer #2 (Recommendations For The Authors):
Regarding the specificity arguments (see above in public review), the authors note that NEMO is very important in IKK specificity, and - if I'm understanding correctly - most of these assays were performed without NEMO. Would the IKK2-NEMO complex change these conclusions?
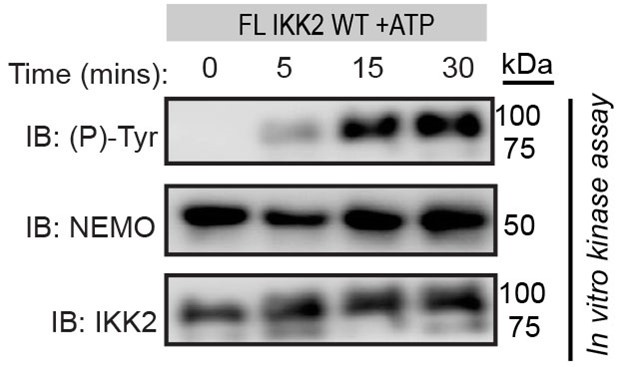
NEMO is a scaffolding protein whose action goes beyond the activation of the IKK-complex. In cells, NEMO brings IkBa from a pool of thousands of proteins to its bonafide kinase when the cells encounter specific signals. In other words, NEMO channels IKK-activity towards its bonafide substrate IkBa at that moment. Though direct proof is lacking, it is likely that NEMO present IkBa in the correct pose to IKK such that the S32/S36 region of IkBa is poised for phosphorylation. The proposed mechanism in the current study further ensures the specificity and fidelity of that phosphorylation event. We believe this mechanism will be preserved in the IKK-NEMO complex unless proven otherwise. As shown below, IKK2 undergoes tyrosine autophosphorylation in presence of NEMO.
Author response image 1.
The work primarily focuses on Y169 as a candidate target for IKK autophosphorylation. This seems reasonable given the proximity to the ATP gamma phosphate. However, Y188F more potently disrupted IκBα phosphorylation. The authors note that this could be due to folding perturbations, but this caveat would also apply to Y169F. A test for global fold perturbations for both Tyr mutants would be helpful.
Y188 is conserved in S/T kinases and that in PKA (Y204) has been studied extensively using structural, biochemical and biophysical tools. It was found in case of PKA that Y204 participates in packing of the hydrophobic core of the large lobe. Disruption of this core structure by mutation allosterically affect the activity of the kinase. We also observed similar engagement of Y188 in IKK2’s large lobe, and speculated folding perturbations in analogy with the experimental evidence observed in PKA. What we meant was mutation of Y188 would allosterically affect the kinase activity. Y169 on the other hand is unique at that position, an no experimental evidence on the effect of phospho-ablative mutation of this residue exist in the literature. Hence, we refrained from speculating its effect on the folding or conformational allostery, however, such a possibility cannot be ruled out.
I struggled to follow the rationalization of the results of Figure 4D, the series of phosphorylation tests of Y169F against IκBα with combinations of phosphoablative or phosphomimetic variants at Ser32 and Ser36. This experiment is hard to interpret without a direct comparison to WT IKK2.
We agree with the reviewer’s concerns. Through this experiment we wanted to inform about the importance of Tyr-phosphorylation of IKK2 in phosphorylating S32 of IκBα which is of vital importance in NF-kB signaling. We have now provided a comparison with WT-IKK2 in the supplementary Figure S3F. We hope this will help bring more clarity to the issue.
MD simulations were performed to compare structures of unphosphorylated vs. Ser-phosphorylated (p-IKK2) vs. Ser+Tyr-phosphorylated (P-IKK2) forms of IKK2. These simulations were performed without ATP bound, and then a representative pose was subject to ADP or ATP docking. The authors note distortions in the simulated P-IKK2 kinase fold and clashes with ATP docking. Given the high cellular concentration of ATP, it seems more logical to approach the MD with the assumption of nucleotide availability. Most kinase domains are highly dynamic in the absence of substrate. Is it possible that the P-IKK2 poses are a result of simulation in a non-physiological absence of bound ATP? Ultimately, this MD observation is linked to the proposed model where ADP-binding is required for efficient phospho-relay to IκBα. Therefore, this observation warrants scrutiny. Perhaps the authors could follow up with binding experiments to directly test whether P-IKK2 binds ADP and fails to bind ATP.
We thank that reviewer for bringing up this issue. This is an important issue and we must agree that we don’t fully understand it yet. We took more rigorous approach this time where we used three docking programs: ATP and ADP were docked to the kinase structures using LeDock and GOLD followed by rescoring with AutoDock Vina. We found that ATP is highly unfavourable to P-IKK2 compared to ADP. To further address these issues, we performed detailed MM-PBSA (Molecular Mechanics Poisson-Boltzmann Surface Area) analyses after MD-simulation to estimate binding free energies and affinities of ADP and ATP for each of the three differently phosphorylated states of IKK2. These analyses (Figure S4 E and F) clearly indicate that phosphorylated IKK2 have much higher preference for ADP over ATP. However, it does not negate ATP-binding by P-IKK2 in a different pose that may not support kinase activity.
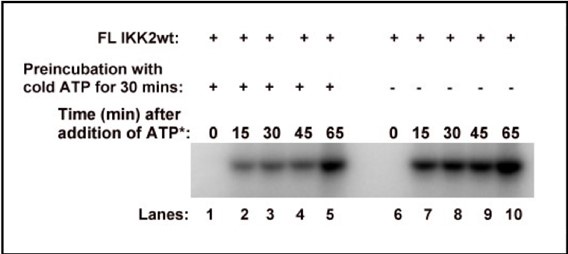
We could not perform any binding experiment because of the following reason. We incubated FL IKK2 WT with or without cold ATP for 30mins, and then incubated these samples with 32P-ATP and analysed the samples by autoradiography after resolving them on a 10% SDS-PAGE. We found that even after pre-incubation of the kinase with excess cold ATP it still underwent autophosphorylation when radioactive ATP was added as shown below. This prevented us from doing direct binding experiment with ATP as it would not represent true binding event. We also noticed that after removal of bulk ATP post autophosphorylation, phosphorylated IKK2 is capable of further autophosphorylation when freshly incubated with ATP. We have not been able to come up with a condition that would only account for binding of ATP and not hydrolysis.
Author response image 2.
The authors could comment on whether robust phosphorylation of NEMO was expected (Figure 1D). On a related note, why is NEMO a single band in the 1D left panel and double bands on the right?
No, we did not expect robust phosphorylation of NEMO. However, robust phosphorylation of NEMO is observed only in the absence of IκBα. In presence of IκBα, phosphorylation of NEMO goes down drastically. These were two different preparations of NEMO. When TEV-digestion to remove His-tag is incomplete it gives two bands as the tagged and untagged versions cannot be separated in size exclusion chromatography which is the final step.
Page 14, line 360. "...observed phosphorylation of tyrosine residue(s) only upon fresh ATP-treatment..." I'm not sure I understand the wording here (or the relevance of the citation). Is this a comment on unreported data demonstrating the rapid hydrolysis of the putative phosphotyrosine(s)? If so, that would be helpful to clarify and report in the supporting information.
In our X-ray crystallographic studies with phosphorylated IKK2 we failed to observe any density of phosphate moiety. Furthermore, this IKK2 showed further autophosphorylation when incubated with fresh ATP. These two observations lead us to believe that some of the autophosphorylation are transient in nature. However, quantitative kinetic analyses of this dephosphorylation have not been performed.
Figure S3 middle panel: The PKA substrate overlaid on the IKK2 seems sterically implausible for protein substrate docking. Is that just a consequence of the viewing angle? On a related note, Figure S3 may be mislabeled as S4 in the main text).
It is a consequence of the viewing angle. Also, we apologize for this inadvertent mislabelling. It has been corrected in the current version.
Reviewer #3 (Recommendations For The Authors):
The detection of phosphorylated amino acids relies largely on antibodies which can have a varying degree of specificity. An alternative detection mode of the phospho-amino acids for example by MS would strengthen the evidence.
We agree with the concern of specificity bias of antibodies. We tried to minimize such bias by using two different p-Tyr antibodies as noted previously and also in the methodology section. We were also able to detect phospho-tyrosine residues by MS/MS analyses, representative spectra are now added (Figure S3A).
IKK2 purity - protocol states "desired purity". What was the actual purity and how was it checked? MS would be useful to check for the presence of other kinases.
Purity of the recombinantly purified IKK2s are routinely checked by silver staining. A representative silver stained SDS-PAGE is shown (Figure S1C). It may be noted that, there’s a direct correlation of expression level and solubility, and hence purification yield and quality with the activity of the kinase. Active IKK2s express at much higher level and yields cleaner prep. In our experience, inactive IKKs like K44M give rise to poor yield and purity. We analysed K44M by LC MS/MS to identify other proteins present in the sample. We did not find any significant contaminant kinase the sample (Figure S1D). The MS/MS result is attached.
Figure 1C&D: where are the Mw markers? What is the size of the band? What is the MS evidence for tyrosine phosphorylation?
We have now indicated MW marker positions on these figures.
MS/MS scan data for the two peptides containing pTyr169 and pTyr188 are shown separately (Figure S3A).
Figure 2F: Why is fresh ATP necessary? Why was Tyr not already phosphorylated? The kinetics of this process appear to be unusual when the reaction runs to completion within 5 minutes ?
As stated earlier, we believe some of the autophosphorylation are transient in nature. We think the Tyr-phosphorylation are lost due to the action of cellular phosphatases. We agree with the concern of the reviewer that, the reaction appears to reach completion within 5 minutes in Fig 2F. We believe it is probably due to the fact that the amount of kinase used in this study exceeds the linear portion of the dynamic range of the antibody used. Lower concentration of the kinase do show that reaction does not reach completion until 60mins as shown in Fig. 2A.
Figure 3: Can the authors exclude contamination with a Tyr kinase in the IKK2-K44M prep? The LC/MS/MS data should be included.
We have reanalysed the sample on orbitrap to check if there’s any Tyr-kinase or any other kinase contamination. We used Spodoptera frugiperda proteome available on the Uniprot website for this analysis. These analyses confirmed that there’s no significant kinase contaminant present in the fraction (Figure S1D).
What is the specificity of IKK-2 Inhibitor VII? Could it inhibit a contaminant kinase?
This inhibitor is highly potent against IKK2 and the IKK-complex, and to a lesser extent to IKK1. No literature is available on its activity on other kinases. In an unrelated study, this compound was used alongside MAPK inhibitor SB202190 wherein they observed completely different outcomes of these two inhibitors (Matou-Nasri S, Najdi M, AlSaud NA, Alhaidan Y, Al-Eidi H, Alatar G, et al. (2022) Blockade of p38 MAPK overcomes AML stem cell line KG1a resistance to 5-Fluorouridine and the impact on miRNA profiling. PLoS ONE 17(5):e0267855. https://doi.org/10.1371/journal.pone.0267855). This study indirectly proves that IKK inhibitor VII does not fiddle with the MAPK pathways. We have not found any literature on the non-specific activity of this inhibitor.
Figure 6B: the band corresponding to "p-IkBa" appears to be similar in the presence of ADP (lanes 4-7) or in the absence of ADP but the presence of ATP (lane 8).
Radioactive p-IκBα level is more when ADP is added than in absence of ADP. In presence of cold ATP, radioactive p-IκBα level remains unchanged. This result strongly indicate that the addition of phosphate group to IκBα happens directly from the radioactively labelled kinase that is not competed out by the cold ATP.





