Author response:
The following is the authors’ response to the original reviews.
Public Reviews:
Reviewer #1 (Public Review):
Summary:
The researchers demonstrated that when cytokine priming is combined with exposure to pathogens or pathogen-associated molecular patterns, human alveolar macrophages and monocyte-derived macrophages undergo metabolic adaptations, becoming more glycolytic while reducing oxidative phosphorylation. This metabolic plasticity is greater in monocyte-derived macrophages than in alveolar macrophages.
Strengths:
This study presents evidence of metabolic reprogramming in human macrophages, which significantly contributes to our existing understanding of this field primarily derived from murine models.
Weaknesses:
The study has limited conceptual novelty.
We acknowledge that the study has limited conceptual novelty, however, the current manuscript provides the field with evidence of the changes in the phenotype and functions of human macrophages in response to IFN-γ or IL-4 which is currently lacking in the literature. Moreover, our data shows for the first time that human airway macrophages change their function in response to IFN-γ.
Reviewer #2 (Public Review):
Summary:
The authors aimed to functionally characterize primary human airway macrophages and monocytederived macrophages, correlating their glycolytic shift in metabolism. They conducted this macrophage characterization in response to type II interferon and IL-4 priming signals, followed by different stimuli of irradiated Mycobacterium tuberculosis and LPS.
Strengths:
(1) The study employs a thorough measurement of metabolic shift in metabolism by assessing extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) of differentially polarized primary human macrophages using the Seahorse XFe24 Analyzer.
(2) The effect of differential metabolic shift on the expression of different surface markers for macrophage activation is evaluated through immunofluorescence flow cytometry and cytokine measurement via ELISA.
(3) The authors have achieved their aim of preliminarily characterizing the glycolysis-dependent cytokine profile and activation marker expression of IFN-g and IL-4 primed primary human macrophages.
(4) The results of the study support its conclusion of glycolysis-dependent phenotypical differences in cytokine secretion and activation marker expression of Ams and MDMs.
Weaknesses:
(1) The data are presented in duplicates for cross-analyses.
(2) The data presented supports a distinct functional profile of airway macrophages (Ams) compared to monocyte (blood)-derived macrophages (MDMs) in response to the same priming signals. However, the study does not attempt to explore the underlying mechanism for this difference.
(3) The study is descriptive in nature, and the results validate IFN-g-mediated glycolytic reprogramming in primary human macrophages without providing mechanistic insights.
(1) We acknowledge the data is presented in duplicate for cross-analyses. This duplication allowed us to examine both (A) the effect of IFN-γ or IL-4 on primary human airway and monocyte derived macrophages in the presence or absence of distinct stimulations and (B) to directly compare the fold change in function occurring in the AM with the changes in the MDM.
(2 & 3) We acknowledge that our study is descriptive however, by inhibiting glycolysis using 2DG we have demonstrated that increased flux through glycolysis is mechanistically required to mediate enhanced cytokine responses in both primary human AM and MDM primed with IFN-γ. However, we acknowledge that we have not determined the differential molecular mechanisms downstream of IFNγ in the AM versus the MDM. IFN-γ promotes both pro- and anti-inflammatory cytokines in AM and this was reduced by inhibiting glycolysis with 2DG. This identifies glycolysis as a key mechanistic pathway which can be therapeutically targeted in AM to modulate inflammation. Mechanistic studies on human AM are limited due to low number of AM retrieved from BAL samples. Nevertheless, the differences between AM and MDM identified in the current study indicate that future mechanistic studies are warranted to identify why IFN-γ promotes IL-10 in AM and not MDM, and, why TNF is differentially regulated by glycolysis in the two macrophage subpopulations, for example.
Reviewer #3 (Public Review):
Summary:
In this manuscript, the authors explore the contribution of metabolism to the response of two subpopulations of macrophages to bacterial pathogens commonly encountered in the human lung, as well as the influence of priming signals typically produced at a site of inflammation. The two subpopulations are resident airway macrophages (AM) isolated via bronchoalveolar lavage and monocyte-derived macrophages (MDM) isolated from human blood and differentiated using human serum. The two cell types were primed using IFNγ and Il-4, which are produced at sites of inflammation as part of initiation and resolution of inflammation respectively, followed by stimulation with either irradiated Mycobacterium tuberculosis (Mtb) or LPS to simulate interaction with a bacterial pathogen. The authors use human cells for this work, which makes use of widely reported and thoroughly described priming signals, as well as model antigens. This makes the observations on the functional response of these two subpopulations relevant to human health and disease. To examine the relationship between metabolism and functional response, the authors measure rates of oxidative phosphorylation and glycolysis under baseline conditions, primed using IFNγ or IL-4, and primed and stimulated with Mtb or LPS.
Strengths:
• The data indicate that both populations of macrophages increase metabolic rates when primed, but MDMs decrease their rates of oxidative phosphorylation after IL-4 priming and bacterial exposure while AMs do not.
• It is demonstrated that glycolysis rates are directly linked to the expression of surface molecules involved in T-cell stimulation and while secretion of TNFα in AM is dependent on glycolysis, in MDM this is not the case. IL-1β is regulated by glycolysis only after IFN-γ priming in both MDM and AM populations. It is also demonstrated that Mtb and LPS stimulation produces responses that are not metabolically consistent across the two macrophage populations. The Mtb-induced response in MDMs differed from the LPS response, in that it relies on glycolysis, while this relationship is reversed in AMs. The difference in metabolic contributions to functional outcomes between these two macrophage populations is significant, despite acknowledgement of the reductive nature of the system by the authors.
• The observations that AM and MDM rely on glycolysis for the production of cytokines during a response to bacterial pathogens in the lung, but that only MDM shift to Warburg Metabolism, though this shift is blocked following exposure to IL-4, are supported by the data and a significant contribution the study of the innate immune response.
Weaknesses:
• It is unclear whether changes in glycolysis and oxidative phosphorylation in primed cells are due to priming or subsequent treatments. ECAR and OCR analyses were therefore difficult to interpret.
All data sets have been presented and analysed relative to both unprimed unstimulated to show both the effect of priming and subsequent stimulation. A second analysis was subsequently conducted where each data set was normalised to its own baseline in terms of percentage change. Therefore, each of unprimed, IFN-γ and IL-4 primed cells were set to 100% in order to assess the effect of stimulation independent of the baseline priming effect. For clarity we have removed the following line:
“Percentage change for ECAR and OCR was calculated from the respective baseline of each data set to visualise the differential ability of IFN-γ, IL-4 primed or unprimed AM to respond to stimulation (Figure S1C,D).”
We have amended the text in the manuscript (lines 164-173) to “Since IFN-γ priming increased cellular energetics in the AM at baseline, we calculated percent change in ECAR and OCR from the baseline rate of each group in order to assess if IFN-γ or IL-4 primed AM have altered capacity to change their metabolism in response to stimulation (Figure 1C,D). This was carried out to equalise all the primed data sets at baseline before stimulation (Figure S1C, S1D). These data indicate that whilst the peak of glycolysis is elevated in IFN-γ primed AM (Figure 1A), all AM have a similar capacity to increase glycolysis upon stimulation when baseline differences in metabolism were adjusted for the effects of cytokine priming (Figure 1C). IFN-γ increased the percent change in OCR of AM in response to both bacterial stimuli compared to the unstimulated IFN-γ primed control (Figure 1D). These data indicate that priming AM alters the metabolic baselines of human tissue resident macrophages and not their ability to respond to bacterial stimuli.”
• The data may not support a claim that AM has greater "functional plasticity" without a direct comparison of antigen presentation. Moreover, MDM secrete more IL-1β than AM. The claim that AM "have increased ability to produce all cytokines assayed in response to Mtb stimulation" does not appear to be supported by the data.
Our data suggests that the MDM are more phenotypically plastic (in terms of their ability to alter expression of cell surface markers in response to cytokine cues), whereas AM have a greater ability to alter cytokine production, our measure of functional plasticity. We have now defined the use of the terms ‘functional plasticity’ and ‘phenotypic plasticity’ in the context of our paper in lines 6063. To consider different culture and plating requirements of MDM versus AM, cytokine production was analysed relative to the average of the unprimed Mtb or LPS control of the respective MDM or AM. This allowed us to draw more accurate comparisons between the two macrophage populations by examining their relative ability to increase their cytokine production (expressed as fold change) rather than defining this functional plasticity only in terms of concentrations of cytokine produced in culture.
We have therefore added the following sentence into the conclusion of the manuscript. “Cumulatively, the data presented herein suggests that the MDM maybe more phenotypically plastic than the AM, while the AM have enhanced functional plasticity in their ability to modulate cytokine production after exposure Th1 and Th2 cytokines.”
We have edited the discussion (lines 421-423) to clarify the following "have increased ability to produce all cytokines assayed in response to Mtb stimulation" and changed it to “stimulated with Mtb have significantly more production of IL-1β, TNF and IL-10 compared with unprimed controls. This is in contrast with IFN-γ primed MDM which only upregulate TNF compared to their unprimed controls.”
• The claim that AM are better for "innate training" via IFNγ may not be consistent with increased IL1β and a later claim that MDM have increased production and are "associated with optimal training."
We have removed the word “better” and now simply state that AM are a tractable target to induce innate training in the human lung.
• Statistical analyses may not appropriately support some of the conclusions.
We have consulted with a statistician. Please see response to reviewer 3 recommendations for authors point 1 below.
• AM populations would benefit from further definition-presumably this is a heterogenous, mixed population.
AM are routinely >97% CD68+CD14+ used in the current study (Author response image 1). However, we acknowledge that tissue resident macrophages represent a spectrum of phenotypes. Given limitations in cell numbers from primary human AM derived from BALF, we have not attempted to define the function of discreet subpopulations of AM.
• The term "functional plasticity" could also be more stringently defined for the purposes of this study.
We are terming functional plasticity to be the macrophages’ ability to alter their production of cytokines in response to external cues like IFN-γ and IL-4 whereas phenotypic plasticity is measured based on ability to alter the cell surface expression of activation markers. We have now defined this in the manuscript (lines 60-63).
Author response image 1.
Expression of macrophage markers on AM.
Conclusion:
Overall, the authors succeed in their goals of investigating how inflammatory and anti-inflammatory cytokine priming contributes to the metabolic reprogramming of AM and MDM populations. Their conclusions regarding the relationship between cytokine secretion and inflammatory molecule expression in response to bacterial stimuli are supported by the data. The involvement of metabolism in innate immune cell function is relevant when devising treatment strategies that target the innate immune response during infection. The data presented in this paper further our understanding of that relationship and advance the field of innate immune cell biology.
Recommendations for the authors:
Reviewer #1 (Recommendations For The Authors):
(1) Authors are suggested to provide rationale for their choice of cytokines as IFN-gamma and IL-4. This will be useful for the readers.
We have updated the following sentence (line 44-46) in the manuscript to add more rationale for the choice of IFN-γ and IL-4. “There is a paucity of data on the role of metabolism in response to Th1 or Th2 microenvironments induced by cytokines-such as IFN-γ or IL-4 respectively, in human macrophages, especially in tissue resident macrophages, such as AM.”
(2) Authors have shown the final outcome of metabolic reprogramming in terms of expression of HLADR and CD-40, and cytokine release. What pathways/receptors are activated or associated with IL-4 and IFN-gamma priming as a first line of response?
The relationship between IFN-γ or IL-4 induced expression of CD40 is established in haematological cell lines and fibroblasts as well as APC, with roles for the JAK/STAT pathways and upregulation of IRFs defined (1-3). Similarly, the relationship between exogenous IFN-γ and upregulation of HLA-DR expression on human monocytes or endothelial cells is established (4, 5). Whist our work does not outline the signalling pathways downstream of Th1 or Th2 cytokine priming, we have shown for the first time that glycolysis mechanistically underpins the shift in phenotype and function observed in human macrophages upon priming with IFN-γ or IL-4.
(3) What are the intracellular signals leading to glycolytic shift?
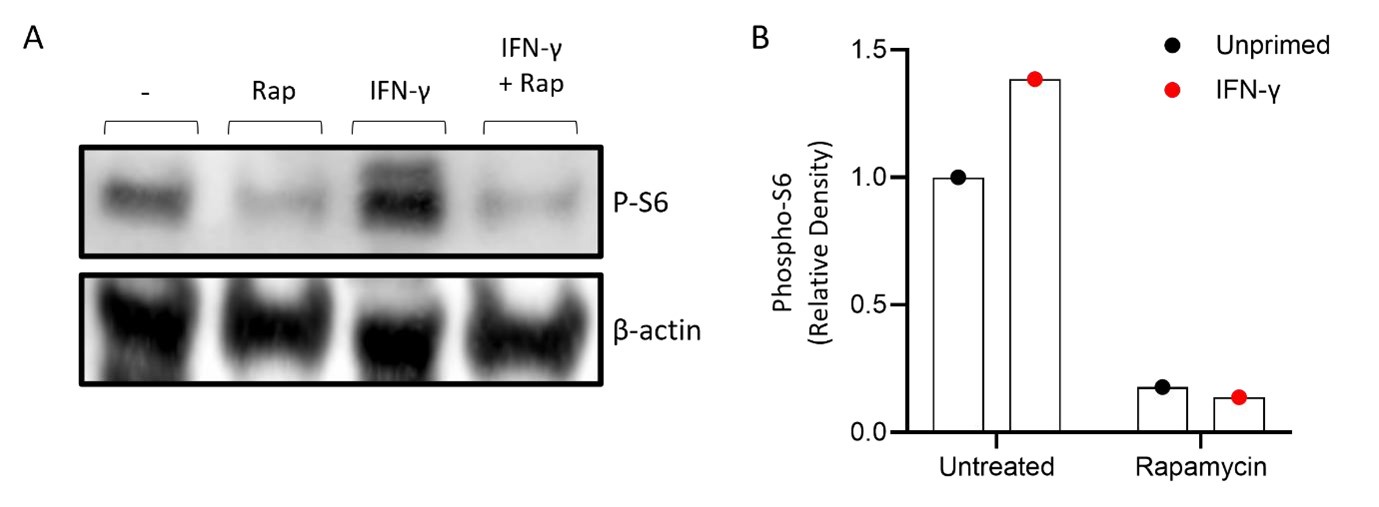
One of the most likely mechanisms that under pin the shift to glycolytic metabolism is the stabilisation of HIF-1α mediated by activation of mTOR (see response below and rebuttal figure 2).
(4) Additional evidence is required to show Warburg effect such as stabilization and activation of HIF1alpha.
We acknowledge that we have not shown the activation and stabilisation of HIF-1α, however, we have provided functional evidence of increased glycolysis with concomitant decreased oxidative phosphorylation indicative of Warburg metabolism.
In order to address this gap in evidence we have reworded the manuscript to describe this functional change to “Warburg-like metabolism” throughout the manuscript. In addition, we have undertaken Western Blotting to provide evidence of mTOR activation when cells are primed with IFN-γ (Author response image 2).
Author response image 2.
IFN-γ activates mTOR in primary human monocytes. Monocytes were isolated from healthy donor PBMC using magnetic separation. Monocytes were left untreated (-), stimulated with rapamycin as a negative control (Rap; 50 nM), IFN-γ (10 ng/ml) or IFN-γ and rapamycin simultaneously (IFN-γ + Rap) for 15 minutes. Phosphorylation of S6 was used as a readout of mTOR activation and measured by western blot using β-actin as a control with a blot (A) and (b) densitometry results are shown as the relative expression of pS6: β-actin from. Graphs show data of n=1 of unprimed (black dot) vs IFN-γ primed (red) with and without rapamycin. ImageLab (Bio-Rad) software was used to perform densitometric analysis.
(5) What is the importance of showing percentage change vs fold change in figure 1 (1C vs 1A)?
All data sets have been presented and analysed relative to both unprimed unstimulated to show the effect of first priming and subsequent stimulation (Figure 1A). A second analysis was subsequently conducted where each data set was normalised to its own baseline in terms of percentage change (Figure 1C). Therefore, each of unprimed, IFN-γ or IL-4 primed cells were set to 100% to assess the effect of stimulation independent of the pre-existing effect of priming on the baseline metabolism. For clarity we have removed the following line:
“Percentage change for ECAR and OCR was calculated from the respective baseline of each data set to visualise the differential ability of IFN-γ, IL-4 primed or unprimed AM to respond to stimulation (Figure S1C,D).”
We have amended the text (lines 164-173) in the manuscript to “Since IFN-γ priming increased cellular energetics in the AM at baseline, we calculated percent change in ECAR and OCR from the baseline rate of each group in order to assess if IFN-γ or IL-4 primed AM have altered capacity to change their metabolism in response to stimulation (Figure 1C,D). This was carried out to equalise all the primed data sets at baseline before stimulation (Figure S1C, S1D). These data indicate that whilst the peak of glycolysis is elevated in IFN-γ primed AM (Figure S1A), all AM have a similar capacity to increase glycolysis upon stimulation when baseline differences in metabolism were adjusted for the effects of cytokine priming (Figure 1C). IFN-γ increased the percent change in OCR of AM in response to both bacterial stimuli compared to the unstimulated IFN-γ primed control (Figure 1D). These data indicate that priming AM alters the metabolic baselines of human tissue resident macrophages and not their ability to respond to bacterial stimuli.”
(6) Why IL-4 primed cells have lower glycolysis than unprimed control cells even in absence of pathogen in Figure 1A?
IL-4 primed AM do not have statistically significant changes in glycolysis compared with unprimed control cells in the absence of stimulation.
Reviewer #2 (Recommendations For The Authors):
The manuscript entitled "Human airway macrophages are metabolically reprogrammed by IFN-γ resulting in glycolysis dependent functional plasticity" by Cox et al., characterizes glycolytic-linked cytokine secretion and surface receptor expression of primary human airway macrophages (AM) and monocyte-derived macrophages (MDM). The authors primed the primary macrophages with type II interferon (IFN-γ) or interleukin-4 (IL-4) into Th1 and Th2 polarized states. This was followed by measurement of the shift in macrophage metabolism to glycolysis (ECAR measurement) and/or oxidative phosphorylation (OCR measurement) in response to lipopolysaccharide and irradiated Mycobacterium tuberculosis. The authors then utilize 2-DG (an inhibitor of glycolysis) to show the reliance of glycolytic shift in metabolism to drive the expression of different macrophage activation markers in MDMs and cytokine secretion in AMs.
Significance:
The study provides important validation of IFN-γ-mediated glycolytic shift and its correlated functionalities in primary human macrophage populations.
Highlights: The study characterizes glycolytic-linked cytokine secretion and expression of macrophage activation markers in primary human resident (lung) and monocyte (blood)-derived macrophages. The study also shows data in support of IFN-γ alone in mediating glycolytic reprogramming of human primary macrophages.
Limitations:
The study lacks novelty and does not provide any new or different information in relation to IFN-γmediated glycolytic shift in the metabolism of human macrophages.
Major comments:
(1) The authors have relied on irradiated Mycobacterium tuberculosis (Mtb) and LPS stimulation to measure different correlates of macrophage functions. Additionally, the authors have discussed their results with irradiated Mtb with that of infection with live Mtb. There are also recent reports that show Mtb infection limiting glycolytic reprogramming in murine and human macrophages (PMID: 31914380) in contrast to their observation with irradiated Mtb. The authors should also include live Mtb infection or other replicative live bacterium for the induction of surface activation markers and cytokine release in their setup.
We thank the reviewer for this suggestion; however, this is beyond the scope of the current study which was to assess AM and MDM in the context of immune stimulation in a reductive manner using TLR4 ligand LPS and a more complete whole bacteria stimulation. The selected bacterial ligands were employed in the study to allow us to model an optimal macrophage host response. This minimises the confounding variable of live bacteria which can perturb cellular metabolism and immune responses, which we have highlighted in the discussion. Since both LPS and irradiated Mtb induced similar metabolic and phenotypic profiles, it is likely that the effects of priming are maintained with diverse stimuli.
(2) The authors should add a quantitative measure (like extracellular lactate secretion or ECAR level) for the extent of glycolytic inhibition by the use of 5 mM 2-DG in their setup.
We would like to draw the attention of the reviewer to the data represented in supplementary figure 2B, demonstrating that 2DG lowers ECAR at 5mM at both 1 and 24 h post stimulation with iH37Rv by an average of approximately 40%. In addition, we have acknowledged that inhibition with 5 mM 2DG does not fully inhibit glycolysis as outlined in the study limitations (lines 477-480).
(3) Percent change and fold change have been used to show the same or similar result in Fig. 1 and 2. Whereas, supplementary Fig. 1 shows absolute ECAR/OCR values in addition to fold change. The authors can plot either fold change or percent change in different measurements to avoid confusion. For example, do ECAR changes upon LPS stimulation in Fig. 1A and 1C come from the same dataset? One of the data points in percent change shows a decrease in percent ECAR change under no cytokine control, whereas all the data points in fold change show an increase.
We have addressed this comment above in response to reviewer 1 point 5 (recommendations for the authors).
We thank the reviewer for highlighting this single error in the data points for percent change. We have fixed this data point which was a result of a calculation error. All data throughout the manuscript has now been rechecked.
Minor comments:
(1) The manuscript for review should be line-marked for referencing and commenting during review.
We have now included line-marking on the manuscript.
(2) The authors can depict marker legends differently for all figures. In all figures, circles to squares or triangles represent treatment/stimulation with iH37Rv or LPS. The authors can depict this as circles to squares/triangles in contrast to different legends.
We have changed the legend to include a more detailed description of data represented inserting additional information regarding the colours and symbols represented in the figures.
(3) Describe bars in supplementary figure 1A - 1H in its legend?
We thank the reviewer for highlighting this oversight, we have amended the legend to state “error bars represent standard deviation”
(4) Discuss the significant increase in CD86 expression in IFN-γ and IL-4 primed unstimulated AMs in Fig. 3E.
We have updated the results section to state that IFN-γ increased the expression of CD86 when isolated in the absence of bacterial stimulations in Fig. 3E (lines 271-272). There is no significant increase in CD86 by IL-4 primed unstimulated AM. IL-4 primed human AM only upregulated CD86 when treated with 2DG or in the presence of stimulation.
(5) Contrary to Fig. 2, the data points of unstimulated cells in Fig. 4 vary for different treatment conditions (no cytokine, IFN-γ, and IL-4) for each cytokine measurement. What is the difference between unstimulated cells in Fig. 4 (for each cytokine) from that of Fig. 2 (for each receptor MFI)?
Unstimulated cells change their surface activation markers and phenotype in response to IFN-γ and IL-4 in Fig. 2. For Fig. 4, IFN-γ and IL-4 are not sufficient to induce cytokine secretion in the absence of stimulation with bacterial ligands.
(6) The methodology for seeding and treatment of cells is reemphasized for almost all results. Defining macrophage priming and stimulation of macrophages in the method section and once at the start of results should be fine.
Plating happens differently for Seahorse compared to the flow cytometric phenotyping and ELISA for cytokine production. For clarity we have stated and reemphasized the seeding and treatment of cells throughout the results section.
(7) Clarify "IL-4 reduced glycolysis in response to LPS stimulation" in relation to the results depicted in Fig. 1A and 1C. Similarly, clarify "IL-4 resulting in reduced IL-1β and IL-10 production" in relation to Fig. 4E.
For clarity we have added the following lines (157-160, 164-170) to the manuscript:
“IL-4 primed iH37Rv stimulated AM increased ECAR to similar extent as unprimed controls (Figure 1A; left). Conversely, IL-4 primed AM stimulated with LPS AM did not increase their ECAR to the same extent as controls (Figure 1A; right), suggesting that IL-4 reduces the AM ability to increase ECAR in response to LPS stimulation.”
“Since IFN-γ priming increased cellular energetics in the AM at baseline, we calculated percent change in ECAR and OCR from the baseline rate of each group in order to assess if IFN-γ or IL-4 primed AM have altered capacity to change their metabolism in response to stimulation (Figure 1C,D). This was carried out to equalise all the primed data sets at baseline before stimulation (Figure S1C, S1D). These data indicate that whilst the peak of glycolysis is elevated in IFN-γ primed AM (Figure S1A), all AM have a similar capacity to increase glycolysis upon stimulation when baseline differences in metabolism were adjusted for the effects of cytokine priming (Figure 1C).”
For clarity we have amended the sentence the reviewer has highlighted (lines 214-215): “IL-4 primed AM had reduced fold change in glycolysis upon stimulation with LPS compared with controls”.
Since IFN-γ priming induced large effect sizes, we statistically analysed the IL-4 primed and unprimed data sets in the absence of the IFN-γ primed data sets to determine how IL-4 influenced macrophage function. The only data where this resulted in any statistical significance was in response to cytokine production. We have now clarified this in the methods and relevant figure legends by stating, “Statistically significant differences were determined using two-way ANOVA with a Tukey post-test (AD); *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001 or #P≤0.05, ##P≤0.01 (where IFN-γ primed data sets were excluded for post-test analysis to analyse statistical differences between no cytokine and IL4 treated data sets).
To further clarify this, we have amended the text of the manuscript (lines 307-310) to reflect this. “All stimulated AM secreted IL-10 regardless of priming (Figure 4E). IFN-γ significantly enhanced iH37Rv induced IL-10 in AM compared to unprimed or IL-4 primed comparators (Figure 4E). IL-4 priming of human AM significantly reduced IL-10 production in response to iH37Rv compared with unprimed AM (Figure 4E). LPS strongly induced IL-10 production in unprimed MDM, which was significantly attenuated by either IFN-γ or IL-4 priming (Figure 4F).”
(8) Clarify whether data points in unstimulated, iH37Rv stimulated, and LPS-stimulated control cells in Fig. 3A - 3F are from independent experiments from those in Fig. 2A - 2F? The distribution of data points of control (no 2-DG treatment) in Fig. 3 is highly similar to the corresponding data points in Fig. 2. Similarly, provide clarification for similarity in Fig. 5A - 5F and Fig. 4A - 4F.
The data illustrated in figure 2 and 3 are from one very large dataset, as are the data in figures 4 and 5. This large experiment was designed to test the effect of priming macrophages with IFN- or IL-4 (in the presence or absence of stimulation), and also to determine if the differential responses elicited due to priming were dependent on glycolysis (by inhibiting with 2DG). For clarity and transparency, the same stimulated dataset is repeated in both figures. Given the size and complexity of the experiment, we chose to present the data this way to aid the reader.
(9) Clarify the statement "where data was reanalyzed in the absence of IFN-γ" in the section pertaining to Statistical analysis. The authors should clearly mention nature of biological and technical replicates for each experiment in its figure legend. The authors should also confirm multiple comparison correction in all 2-way ANOVA tests done in each figure legend."
We have amended the text (lines 133-136) to clarify this point “P-values of ≤0.05 were considered statistically significant and denoted with an asterisk. Alternatively, P-values of ≤0.05 were denoted with a hashtag where data was analysed in the absence of IFN-γ primed data sets, to analyse statistical differences between no cytokine and IL-4 treated data sets.”
Figures represent biological replicates (which are the average of technical replicates, presented as a single data point). This is indicated by the following sentence in each figure legend: “Each linked data point represents the average of technical duplicates for one individual biological donor”.
Each legend has been amended to include the multiple comparison post-test applied.
(10) Discuss the differences and similarities of IFN-γ driven metabolic reprogramming of primary murine macrophages with the results of this study relative to cytokine secretion and activation marker expression.
We have added additional discussion and detail comparing human and murine macrophages in lines 381-382, 403, 407 and 412-415 of the manuscript.
(11) The repetitive data plots of similar results can be significantly reduced to improve the interpretation of the results.
The benefit of the plotting the data in this way is for a clearer understanding and representation of the data. The repetitive data plots allow the benefit of being able to first delineate the effect of priming and priming plus stimulation and then, separately, to further examine the differences in AM versus MDM. The repetition of the primed data points then allows of the reader to determine the effect of inhibiting glycolysis with 2DG on unprimed and primed macrophages (with and without stimulation).
Reviewer #3 (Recommendations For The Authors):
The methods used and data reported in this manuscript contribute to our understanding of the role of metabolism in programming of macrophages during priming. Suggestions for improving the presentation and interpretation of results include:
• Consult with a statistician regarding analyses of the multiple conditions used during these assays. The use of repeated statistical analyses with different comparison groups in the same figure/data set seems atypical and should either be amended or fully justified in the text. Also, use of two-way vs. one-way ANOVA should be evaluated and clarified.
We have now consulted a statistician. We have amended the text (lines 133-136) to clarify this point “P-values of ≤0.05 were considered statistically significant and denoted with an asterisk. Alternatively, P-values of ≤0.05 were denoted with a hashtag where data was analysed in the absence of IFN-γ primed data sets, to analyse statistical differences between no cytokine and IL-4 treated groups.”
There are two variables in the data sets; cytokine priming as well as stimulation status therefore we opted for a two-way ANOVA rather than a One-way ANOVA. There are three stimulation groups: unstimulated, Mtb-stimulated and LPS-stimulated. Cytokine priming also has three groups: no cytokine, IFN-y, or IL-4. There are two variables (priming and stimulation), each with 3 groups i.e., six treatment conditions in total, therefore two-way AVOVA with multiple comparisons tests help pinpoint exactly which groups (e.g., the 6 different levels of the 'stimulation' and 'cytokine' treatments) are significantly different from each other. This was important for understanding the specific effects of our treatments. The reader can therefore also deduce how these six treatment conditions compare to each other.
In contrast, performing multiple single comparisons independently of the rest of the dataset (e.g. t tests), increases the risk of false positives (type 1 error). Multiple comparisons ANOVA with post-tests adjust for this, helping to reduce the likelihood of a type 1 error. These stats are more stringent, and it is therefore harder to get P values <0.05. Hence, if we compared all six treatment groups without adjustment, you increase the chance of finding false positives due to the sheer number of comparisons, leading to biased and incorrect conclusions.
In our case, multiple comparisons tests were essential after the two-way ANOVA because they helped to objectively identify specific treatment group differences and control the overall error rate when we were extracting our conclusions, thereby reducing any risk of biases in our conclusions.
A one-way ANOVA is used to test the effect of a single variable with more than two groups contained in the dataset. For example, in our case if you only want to test how different 'stimulation' groups affect ECAR or OCR, only in unprimed macrophages, a one-way ANOVA would be used.
The current study used two-way ANOVA to test the effects of two variables (priming and stimulation, or in some cases priming and inhibition) each containing 3 groups, and see if there is any interaction between the two factors. For example, in our case this allowed us to examine how the 'stimulation' and the 'cytokine' priming affect ECAR/OCR levels and to determine if the effect of 'stimulation' depends on the 'cytokine' priming.
• More justification could be given for the dose of IFNγ used for priming. Inflammatory priming is typically performed with a "low-dose" treatment (e.g., ~1 ng/ml), whereas the authors use 10 ng/ml, which would be considered a high dose. It would be useful to repeat select experiments with a more standard low-dose treatment of IFNg to demonstrate that this is also sufficient to induce the observed metabolic changes.
Previous work has identified little difference in the response of AM and peripheral monocytes to low versus high doses of IFN-γ (6). We have inserted the following into the study limitations (lines 479-481).
“Furthermore, only one dose of IFN-γ was utilised due to limitations in AM yield, however, recently both low and high doses of IFN-γ have been shown to have similar effects on AM in vitro (6).”
• Check for accuracy of the Fig.4 legend. Also check that 4G and 4B math is consistent.
The legend for Figure 4 has been amended for incorrect A,B to state G,H. The math has been double checked for accuracy and is correct. 3 out of 10 MDM donors produced IL-1β in the absence of IFN-γ in Figure 4B, therefore the average used to calculate the data represented in Figure 4G was brought down markedly by donors who produced little or no IL-1β.
• Functional plasticity is a vague term and difficult to interpret in this context. It is stated that AM have greater functional plasticity, but MDMs appear to have greater capacity to secrete IL-1β and respond more robustly to IL-4 in terms of T cell stimulation. On that note, the claims regarding antigen presentation would be more impactful if a direct comparison of antigen presentation capacity was made between AM and MDM.
Our data suggests that AM have a greater ability to alter cytokine production, such as IL1β. To consider different culture and plating requirements of MDM v AM cytokine concentration was normalised and expressed in terms of fold change. This gives a more controlled and accurate comparison of the ability of IFN-γ or IL-4 to modulate cytokine production in AM compared with MDM.
The terms ‘functional plasticity’ and phenotypic plasticity’ have now been defined in the manuscript in lines 60-63.
We have therefore added the following sentence into the conclusion of the manuscript (lines 490-493). “Cumulatively, the data presented herein suggests that the MDM maybe more phenotypically plastic than the AM, while the AM have enhanced functional plasticity in their ability to produce cytokine after exposure Th1 and Th2 cytokines.”
However, we acknowledge that the MDM may be regarded as more plastic because of their ability to respond robustly to IL-4, whereas the phenotypic and functional changes in the AM in response to IL4 are more limited. Whilst the focus of our work was to determine if AM are a tractable target to promote immunity in the lungs through upregulation of pro-inflammatory effector function, their ability to downregulated inflammation in response to IL-4 is comparatively less profound compared with MDM.
We acknowledge the shortcomings of our work which did not allow us to directly measure antigen processing in the AM, due to limitations in the cellular yield from BALF. We have edited the text (lines 251-252 and 286) to clarify this for the reader.
• Inconsistent normalization complicates interpretation of metabolic data. For example, it is unclear, for example, whether changes in glycolysis and oxidative phosphorylation in primed cells are due to priming or subsequent treatments. Check harmony of methods for analysis of "metabolic assays" with Fig.1 data, axis, and legend.
We have addressed this comment, which is similar to points made by the other reviewers and amended the manuscript to increase clarity. These changes are outlined in the response to reviewer 1, point 5 (recommendations for the author). In addition, we have amended the metabolic assay method (lines 111-112) to state that “Post stimulation the ECAR and OCR were continually sampled at 20-minute intervals for times indicated.”
• A direct comparison of cytokine production after priming and stimulation with Mtb or LPS is limited by inconsistent axes. The data may not support a claim that AM has greater "functional plasticity" without a direct comparison of antigen presentation. Moreover, MDM secrete more IL-1β than AM. The claim that that AM "have increased ability to produce all cytokines assayed in response to Mtb stimulation" does not appear to be supported by the data.
We have amended the text to clarify this issue (lines 313-315). “These data suggest that the AM have greater functional plasticity in terms of their ability to upregulate cytokine production in response to IFN-γ, compared with the MDM. IFN-γ primed AM have enhanced IL-10 and TNF production in response to Mtb and LPS, respectively.”
We have amended the manuscript and have replaced “IFN-γ primed AM have increased ability to produce all cytokines assayed in response to Mtb stimulation” with the following (lines 421-423) “IFNγ primed AM stimulated with Mtb have significantly more production of IL-1β, TNF and IL-10 compared with unprimed controls. This is in contrast with IFN-γ primed MDM which only upregulate TNF compared to their unprimed controls.”
• AM populations could be defined experimentally.
Airway macrophages were adherence purified from bronchoalveolar lavage fluid defined as CD68+CD14+ as per rebuttal figure 1. The purpose of this study was to examine if human peripherally derived or lung resident macrophages were plastic in response to the classical polarising cytokines IFNγ and IL-4. We have identified that the AM and MDM do indeed have different functional and metabolic responses to these cytokines. However, determining functional differences within the AM subpopulations is beyond the scope of the current study and hampered by low cell numbers in human BALF.
References
(1) Conzelmann M, Wagner AH, Hildebrandt A, Rodionova E, Hess M, Zota A, Giese T, Falk CS, Ho AD, Dreger P, Hecker M, Luft T. IFN-γ activated JAK1 shifts CD40-induced cytokine profiles in human antigen-presenting cells toward high IL-12p70 and low IL-10 production. Biochemical pharmacology 2010; 80: 2074-2086.
(2) Fries KM, Sempowski GD, Gaspari AA, Blieden T, Looney RJ, Phipps RP. CD40 Expression by human fibroblasts. Clinical Immunology and Immunopathology 1995; 77: 42-51.
(3) Gu W, Chen J, Yang L, Zhao KN. TNF-α promotes IFN-γ-induced CD40 expression and antigen process in Myb-transformed hematological cells. TheScientificWorldJournal 2012; 2012: 621969.
(4) Hershman MJ, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC, Jr. Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clinical and experimental immunology 1989; 77: 67-70.
(5) Maenaka A, Kenta I, Ota A, Miwa Y, Ohashi W, Horimi K, Matsuoka Y, Ohnishi M, Uchida K, Kobayashi T. Interferon-γ-induced HLA Class II expression on endothelial cells is decreased by inhibition of mTOR and HMG-CoA reductase. FEBS open bio 2020; 10: 927-936.
(6) Thiel BA, Lundberg KC, Schlatzer D, Jarvela J, Li Q, Shaw R, Reba SM, Fletcher S, Beckloff SE, Chance MR, Boom WH, Silver RF, Bebek G. Human alveolar macrophages display marked hyporesponsiveness to IFN-γ in both proteomic and gene expression analysis. PLoS One 2024; 19: e0295312.





