Author response:
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
Strengths:
The three experiments are well designed and the various conditions are well controlled. The rationale of the study is clear, and the manuscript is pleasant to read. The analysis choices are easy to follow, and mostly appropriate.
We are grateful to the reviewer’s thoughtful comments.
Weaknesses:
I only have one potential worry. The analysis for gait tracking (1 Hz) in Experiment 2 (Figures 3a/b) starts by computing a congruency effect (A/V stimulation congruent (same frequency) versus A/V incongruent (V at 1 Hz, A at either 0.6 or 1.4 Hz), separately for the Upright and Inverted conditions. Then, this congruency effect is contrasted between Upright and Inverted, in essence computing an interaction score (Congruent/Incongruent X Upright/Inverted). Then, the channels in which this interaction score is significant (by cluster-based permutation test; Figure 3a) are subselected for further analysis. This further analysis is shown in Figure 3b and described in lines 195-202. Critically, the further analysis exactly mirrors the selection criteria, i.e. it is aimed at testing the effect of Congruent/Incongruent and Upright/Inverted. This is colloquially known as "double dipping", the same contrast is used for selection (of channels, in this case) as for later statistical testing. This should be avoided, since in this case even random noise might result in a significant effect. To strengthen the evidence, either the authors could use a selection contrast that is orthogonal to the subsequent statistical test, or they could skip either the preselection step or the subsequent test. (It could be argued that the test in Figure 3b and related text is not needed to make the point - that same point is already made by the cluster-based permutation test.)
Thanks for the helpful suggestions. In Experiment 2, to investigate whether the multisensory integration effect was specialized for biological motion perception, we contrasted the congruency effect between the upright and inverted conditions to search for clusters showing a significant interaction effect. We performed further analyses based on neural responses from this cluster to examine whether the congruency effect was significant in the upright and the inverted conditions, respectively, following the logic of post hoc comparisons after identifying an interaction effect. However, we agree with the reviewer that comparing the congruency effects between the upright and inverted conditions again based on data from this cluster was redundant and resulted in doubledipping. Therefore, we have removed this comparison from the main text and optimized the way to present our results in the revised Fig. 3).
Related to the above: the test for the three-way interaction (lines 211-216) is reported as "marginally significant", with a p-value of 0.087. This is not very strong evidence.
As shown in Fig.3b & e, the magnitude of amplitude differs between the gaitcycle frequency (mean = 0.008, SD = 0.038) and the step-cycle frequency (mean = 0.052; SD =0.056), which might influence the statistical results of the interaction effect. To reduce such influence, we converted the amplitude data at each frequency condition into Z-scores, separately. The repeated-measures ANOVA analysis on these normalized amplitude data revealed a significant three-way interaction (F (1,23) = 7.501, p = 0.012, ƞp2 = 0.246). We have updated the results in the revised manuscript (lines 218-225).
Reviewer #1 (Recommendations For The Authors):
- Which variable caused one data point to be classified as outlier? (line 221).
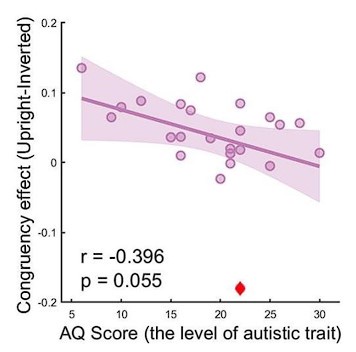
The outlier is a participant whose audiovisual congruency effect (Upright – Inverted) in neural responses at the frequency of interest exceeds 3 SD from the group mean. It is marked by a red diamond in Author response 2. Before removing the data, the correlation between the AQ score and the congruency effect is r = -0.396, p = 0.055. For comparison, the results after removing the outlier are shown in Fig. 3c of the revised manuscript. We have added more information about the variable causing the outlier in the revised manuscript (lines 231-232).
Author response image 1.
The correlation between AQ score and congruency effect
- The authors cite Maris & Oostenveld (2007) in line 415 as the main reference for the FieldTrip toolbox, but the correct reference here is different, see https://www.fieldtriptoolbox.org/faq/how_should_i_refer_to_fieldtrip_in_my_p ublication/
Thank you for pointing out this issue. Citation corrected.
- The authors could consider giving some more background on the additive vs superadditive distinction in the Introduction, which may increase the impact; as it stands the reader might not know why this is particularly interesting. Summarize some of the takeaways of the Stevenson et al. (2014) review in this respect.
Thanks for the suggestion and we have added the following relevant information in the Introduction (lines 80-90):
“Moreover, we adopted an additive model to classify multisensory integration based on the AV vs A+V comparison. This model assumes independence between inputs from each sensory modality and distinguishes among sub-additive (AV < A+V), additive (AV = A+V), and super-additive (AV > A+V) response modes (see a review by Stevenson et al., 2014). The additive mode represents a linear combination between two modalities. In contrast, the super-additive and subadditive modes indicate non-linear interaction processing, either with potentiated neural activation to facilitate the perception or detection of nearthreshold signals (super-additive) or a deactivation mechanism to minimize the processing of redundant information cross-modally (sub-additive) (Laurienti et al., 2005; Metzger et al., 2020; Stanford et al., 2005; Wright et al., 2003).”
Reviewer #2 (Public Review):
Strengths:
The manuscript is well-written, with a concise and clear writing style. The visual presentation is largely clear. The study involves multiple experiments with different participant groups. Each experiment involves specific considered changes to the experimental paradigm that both replicate the previous experiment's finding yet extend it in a relevant manner.
We thank the reviewer for the valuable feedback.
Weaknesses:
The manuscript interprets the neural findings using mechanistic and cognitive claims that are not justified by the presented analyses and results.
First, entrainment and cortical tracking are both invoked in this manuscript, sometimes interchangeably so, but it is becoming the standard of the field to recognize their separate evidential requirements. Namely, step and gate cycles are striking perceptual or cognitive events that are expected to produce event-related potentials (ERPs). The regular presentation of these events in the paradigm will naturally evoke a series of ERPs that leave a trace in the power spectrum at stimulation rates even if no oscillations are at play. Thus, the findings should not be interpreted from an entrainment framework except if it is contextualized as speculation, or if additional analyses or experiments are carried out to support the assumption that oscillations are present. Even if oscillations are shown to be present, it is then a further question whether the oscillations are causally relevant toward the integration of biological motion and for the orchestration of cognitive processes.
Second, if only a cortical tracking account is adopted, it is not clear why the demonstration of supra-additivity in spectral amplitude is cognitively or behaviorally relevant. Namely, the fact that frequency-specific neural responses to the [audio & visual] condition are stronger than those to [audio] and [visual] combined does not mean this has implications for behavioral performance. While the correlation to autism traits could suggest some relation to behavior and is interesting in its own right, this correlation is a highly indirect way of assessing behavioral relevance. It would be helpful to test the relevance of supra-additive cortical tracking on a behavioral task directly related to the processing of biological motion to justify the claim that inputs are being integrated with the service of behavior. Under either framework, cortical tracking or entrainment, the causal relevance of neural findings toward cognition is lacking.
Overall, I believe this study finds neural correlates of biological motion, and it is possible that such neural correlates relate to behaviorally relevant neural mechanisms, but based on the current task and associated analyses this has not been shown.
Thanks for raising the important concerns regarding the interpretation of our results within the entrainment or the cortical tracking frame. A strict neural entrainment account emphasizes the alignment of endogenous neural oscillations with external rhythms, rather than a mere regular repetition of stimulus-evoked responses. However, it is challenging to fully dissociate these components, given that rhythmic stimulation can shape intrinsic neural oscillations, resulting in an intricate interplay between endogenous neural oscillations and stimulus-evoked responses (Duecker et al., 2024; Herrmann et al., 2016; Hosseinian et al., 2021). Therefore, some research, including the current study, use the term “entrainment” to refer to the alignment of brain activity to rhythmic stimulation in a broader context, without isolating the intrinsic oscillations and evoked responses (e.g., Ding et al., 2016; Nozaradan et al., 2012; Obleser & Kayser, 2019). Nevertheless, we agree with the reviewer that since the current results did not examine or provide direct evidence for endogenous oscillations, it is better to contextualize the oscillation view as speculations. Hence, we have replaced most of the expressions about “entrainment” with a more general term “tracking” in the revised manuscript (as well as in the title of the manuscript). We only briefly mentioned the entrainment account in the Discussion to facilitate comparison with the literature (lines 307-312).
Regarding the relevance between neural findings and cognition or behavioral performance, the first supporting evidence comes from the inversion effect in Experiment 2. For the neural responses at gait-cycle frequency, we observed a significantly enhanced audiovisual congruency effect in the upright condition compared with the inverted condition. Inversion disrupts the distinctive kinematic features of biological motion (e.g., gravity-compatible ballistic movements) and significantly impairs biological motion processing, but it does not change the basic visual properties of the stimuli, including the rhythmic signals generated by low-level motion cues. Therefore, the inversion effect has long been regarded as an indicator of the specificity of biological motion processing in numerous behavioral and neuroimaging studies (Bardi et al., 2014; Grossman & Blake, 2001; Shen, Lu, Yuan, et al., 2023; Simion et al., 2008; Troje & Westhoff, 2006; Vallortigara & Regolin, 2006; Wang et al., 2014; Wang & Jiang, 2012; Wang et al., 2022). Here, our finding of the cortical tracking of higher-order rhythmic structures (gait cycles) present in the upright but not in the inverted condition suggests that this cortical tracking effect can not be explained by ERPs evoked by regular onsets of rhythmic events. Rather, it is closely linked with the specialized cognitive processing of biological motion. Furthermore, we found that the BM-specific cortical tracking effect at gait-cycle frequency (rather than the non-selective tracking effect at step-cycle frequency) correlates with observers’ autistic traits, indicating its functional relevance to social cognition. These findings convergingly suggest that the cortical tracking effect that we currently observed engages cognitively relevant neural mechanisms. In addition, our recent behavioral study showed that listening to frequency-congruent footstep sounds, compared with incongruent sounds, enhanced the visual search for human walkers but not for non-biological motion stimuli containing the same rhythmic signals (Shen, Lu, Wang, et al., 2023). These results suggest that audiovisual correspondence specifically enhances the perceptual and attentional processing of biological motion. Future research could examine whether the cortical tracking of rhythmic structures plays a functional role in this process, which may shed more light on the behavioral relevance of the cortical tracking effect to biological motion perception. We have incorporated the above information into the Discussion (lines 268-293).
Reviewer #2 (Recommendations For The Authors):
In Figure 1c, it could be helpful to add the word "static" in the illustration for the auditory condition so that readers understand without reading the subtext that it is a static image without biological motion.
Suggestion taken.
In the Discussion, I believe it is important to justify an oscillation and entrainment account, or if it cannot be justified based on the current results and analyses (which is my opinion), it could be helpful to explicitly frame it as speculation.
We agree with the reviewer. For more clarification, please refer to our response to the public review.
L335, I did not understand this sentence - a reformulation would be helpful.
The point-light stimuli were created by capturing the motion of a walking actor (Vanrie & Verfaillie, 2004). The global motion of the walking sequences was eliminated so that the point-light walker looks like walking on a treadmill without translational motion. We have reformulated the sentence as follows: “The point-light walker was presented at the center of the screen without translational motion.”
The results in Figure 2a and 2d are derived by performing a t-test between the amplitude at the frequency of gait and step cycles and zero. Comparison against amplitude of zero is too liberal; the possibility for a Type-I error is inflated because even EEG data with only noise will not have amplitudes of zero at all frequencies. A better baseline (H0) is either the 1/frequency trend in the power spectrum derived using methods like FOOOF (https://fooof-tools.github.io/fooof/) or by performing non-parametric shuffling based methods (https://doi.org/10.1016/j.jneumeth.2007.03.024).
In our data analysis, instead of performing the t-test between raw amplitude with zero, we compared the normalized amplitude at each frequency bin (by subtracting the average amplitude measured at the neighboring frequency bins from the original amplitude data) against zero. Such analysis is equal to contrasting the raw amplitude to its neighboring frequency bins, allowing us to test whether the neural response in each frequency bin showed a significant enhancement compared with its neighbors. The multiple comparisons on each frequency bin were controlled by false discovery rate (FDR) correction, reducing the Type-I error. Such analysis procedures help reduce (though not totally remove) the influence of the 1/f trend and have been widely used in this field (Cirelli et al., 2016; Henry & Obleser, 2012; Lenc et al., 2018; Nozaradan et al., 2012; Peter et al., 2023).
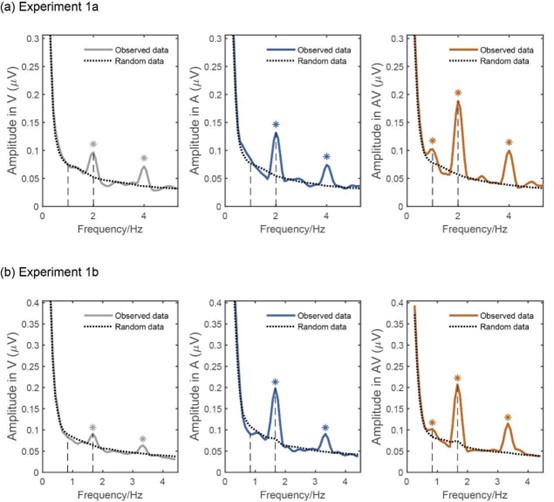
To further verify our findings, we adopted the reviewer’s suggestion and created a baseline by performing a non-parametric shuffling-based analysis. More specifically, to establish the statistical significance of amplitude peaks, we carried out a surrogate analysis on each condition. For each participant, a single control surrogate dataset was derived from their actual dataset by jittering the onset of each step-cycle relative to the actual original onset by a randomly selected integer value ranging between − 490–490 ms. This procedure removed the consistent relationship between the EEG signal and the stimuli while preserving each epoch’s general timing within the exposure period. Then, epochs were extracted based on surrogate stimuli onset, and amplitude was computed across frequencies through FFT under a null model of non-entrainment (Moreau et al., 2022). This entire procedure was performed 100 times, producing a surrogate amplitude distribution of 100 group-averaged values for each condition. If the observed amplitude values at the frequency of interest exceeded the value corresponding to the 95th percentile of the surrogate distribution (p < .05) within a given condition (e.g., AV), the amplitude peak was considered significant (Batterink, 2020). As shown in Author response image 2, the statistical results from these analyses are similar to those reported in the manuscript, confirming the significant amplitude peaks at the frequencies of interest.
Author response image 2.
Non-parametric analysis for spectral peak. The dotted lines represent the random data based on shuffling analysis. The solid lines represent the observed data in measured EEG signals. All conditions induced significant peaks at step-cycle frequency and its harmonic, while only the AV condition induced a significant peak at gait-cycle frequency.
Reviewer #3 (Public Review):
Strengths:
The main strengths of the paper relate to the conceptualization of BM and the way it is operationalized in the experimental design and analyses. The use of entrainment, and the tracking of different, nested aspects of BM result in seemingly clean data that demonstrate the basic pattern. The first experiments essentially provide the basic utility of the methodological innovation and the second experiment further hones in on the relevant interpretation of the findings by the inclusion of better control stimuli sets.
Another strength of the work is that it includes at a conceptual level two replications.
We appreciate the reviewer for the comprehensive review and positive comments.
Weaknesses:
The statistical analysis is misleading and inadequate at times. The inclusion of the autism trait is not foreshadowed and adequately motivated and is likely underpowered. Finally, a broader discussion over other nested frequencies that might reside in the point-light walker stimuli would also be important to fully interpret the different peaks in the spectra.
(1) Regarding the nested frequency peaks in the spectra, we did observe multiple significant amplitude peaks at 1f (1/0.83 Hz), 2f (2/1.67 Hz), and 4f (4/3.33 Hz) relative to the gait-cycle frequency (Fig. 2 a&d). To further test the functional roles of the neural activity at different frequencies, we analyzed the audiovisual integration modes at each frequency. Note that we collapsed the data from Experiments 1a & 1b in the analysis as they yielded similar results. Overall, results show a similar additive audiovisual integration mode at 2f and 4f and a super-additive integration mode only at 1f (Figure S1), suggesting that the cortical tracking effects at 2f and 4f may be functionally linked but independent of that at 1f. We have reported the detailed results in the Supplementary Information.
(2) For the reviewer’s other concerns about statistical analysis and autism traits, please refer to our responses below to the Recommendations for the authors.
Reviewer #3 (Recommendations For The Authors):
The description of the analyses performed for experiment 2 comes across as double dipping. Congruency effects for BM and non-BM motion (inverted) were compared using cluster-based statistics. Then identified clusters informed an averaging of signals which then were subjected to a paired comparison. At this point, it is no surprise that these paired comparisons are highly significant seeing that the channels were selected based on a cluster analysis of the same exact contrast. This approach should be avoided.
In the analysis of the repeated measures ANOVA reporting a trend as marginally significant is misleading. Reporting the statistical results whilst indicating that those do not reach significance is the appropriate way to communicate this finding. Other statistics can be used in order to provide the likelihood of those findings supporting H1 or H0 if the authors would like to state something more precise (Bayesian).
Thanks for the comments. We have addressed these two points in our response to the public review of Reviewer #1.
The authors perform a correlation along "autistic trait" scores in an individual differences approach. Individual differences are typically investigated in larger samples (>n=40). In addition, the range of AQ scores seems limited to mostly average or lower-than-average AQs (barring a couple). These points make the conclusions on the possible role of BM in the autistic phenotype very tentative. I would recommend acknowledging this.
An alternative analysis approach that might better suit the smaller sample size is a comparison between high and low AQ participants, defined based on a median split.
Many thanks for the suggestion. We agree with the reviewer that the sample size (n = 24) in the current study is not large for exploring the correlation between BM and autistic traits. The narrow range of AQ scores was due to the fact that all participants were non-clinical populations and we did not pre-select participants by AQ scores. To further confirm our findings, we adopted your suggestion to compare the BM-specific cortical tracking effect (i.e., audiovisual congruency effect (Upright - Inverted)) between high and low AQ participants split by the median AQ score (20) of this sample. Similar to correlation analysis, one outlier, whose audiovisual congruency effect (Upright – Inverted) in neural responses at 1 Hz exceeds 3 SD from the group mean, was removed from the following analysis. As shown in Figure S3, at 1 Hz, participants with low AQ showed a greater cortical tracking effect compared with high AQ participants (t (21) = 2.127, p = 0.045). At 2 Hz, low and high AQ participants showed comparable neural responses (t (22) = 0.946, p = 0.354). These results are in line with the correlation analysis, providing further support to the functional relevance between social cognition and cortical tracking of biological motion as well as its dissociation at the two temporal scales. We have added these results to the main text (lines 238-244) and the supplementary information.
Writing
The narrative could be better unfolded and studies better motivated. The transition from basic science research on BM to possibly delineating a mechanistic understanding of autism was a surprise at the end of the intro. Once the authors consider the suggestions and comments above it would be good to have this detail and motivation more obviously foreshadowed in the text.
Thanks for the great suggestion and we have provided an introduction about how audiovisual BM processing links with social cognition and ASD in the first paragraph of the revised manuscript (lines 46-56). In particular, integrating multisensory BM cues is foundational for perceiving and attending to other people and developing further social interaction. However, such ability is usually compromised in people with social deficits, such as individuals with autism spectrum disorder (ASD) (Feldman et al., 2018), and even in non-clinical populations with high autistic traits (Ujiie et al., 2015). These behavioral findings underline the close relationship between multisensory BM processing and one’s social cognitive capability, motivating us to further explore this issue at the neural level in the current study. We have also modified the relevant content in the last paragraph of the Introduction (lines 100-108), briefly mentioning the methods that we used to investigate this issue.
The use of terminology related to neural oscillations which are entraining to the BM seems to suggest that the rhythmic tracking inevitably stems from the shaping of existing intrinsic dynamics of the brain. I am not sure this is necessarily the case. I would therefore adopt a more concrete jargon for the description of the entrainment seen in this study. If a discussion over internal dynamics shaped by external stimuli should be invoked, it should be done explicitly with appropriate references (but in my opinion, it isn't quite required).
Please refer to our response to a similar point raised in the public review of Reviewer #2.
References
Bardi, L., Regolin, L., & Simion, F. (2014). The First Time Ever I Saw Your Feet: Inversion Effect in Newborns’ Sensitivity to Biological Motion. Developmental Psychology, 50. https://doi.org/10.1037/a0034678
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/highfunctioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. https://doi.org/10.1023/a:1005653411471
Batterink, L. (2020). Syllables in Sync Form a Link: Neural Phase-locking Reflects Word Knowledge during Language Learning. Journal of Cognitive Neuroscience, 32(9), 1735–1748. https://doi.org/10.1162/jocn_a_01581
Cirelli, L. K., Spinelli, C., Nozaradan, S., & Trainor, L. J. (2016). Measuring Neural Entrainment to Beat and Meter in Infants: Effects of Music Background. Frontiers in Neuroscience, 10. https://doi.org/10.3389/fnins.2016.00229
Ding, N., Melloni, L., Zhang, H., Tian, X., & Poeppel, D. (2016). Cortical tracking of hierarchical linguistic structures in connected speech. Nature Neuroscience, 19(1), 158–164. https://doi.org/10.1038/nn.4186
Duecker, K., Doelling, K. B., Breska, A., Coffey, E. B. J., Sivarao, D. V., & Zoefel, B. (2024). Challenges and approaches in the study of neural entrainment. Journal of Neuroscience, 44(40). https://doi.org/10.1523/JNEUROSCI.1234-24.2024
Falck-Ytter, T., Nyström, P., Gredebäck, G., Gliga, T., Bölte, S., & the EASE team. (2018). Reduced orienting to audiovisual synchrony in infancy predicts autism diagnosis at 3 years of age. Journal of Child Psychology and Psychiatry, 59(8), 872–880. https://doi.org/10.1111/jcpp.12863
Feldman, J. I., Dunham, K., Cassidy, M., Wallace, M. T., Liu, Y., & Woynaroski, T. G. (2018). Audiovisual multisensory integration in individuals with autism spectrum disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 95, 220–234. https://doi.org/10.1016/j.neubiorev.2018.09.020
Grossman, E. D., & Blake, R. (2001). Brain activity evoked by inverted and imagined biological motion. Vision Research, 41(10), 1475–1482. https://doi.org/10.1016/S0042-6989(00)00317-5
Henry, M. J., & Obleser, J. (2012). Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proceedings of the National Academy of Sciences, 109(49), 20095–20100. https://doi.org/10.1073/pnas.1213390109
Herrmann, C. S., Murray, M. M., Ionta, S., Hutt, A., & Lefebvre, J. (2016). Shaping Intrinsic Neural Oscillations with Periodic Stimulation. Journal of Neuroscience, 36(19), 5328–5337. https://doi.org/10.1523/JNEUROSCI.0236-16.2016
Hosseinian, T., Yavari, F., Biagi, M. C., Kuo, M.-F., Ruffini, G., Nitsche, M. A., & Jamil, A. (2021). External induction and stabilization of brain oscillations in the human. Brain Stimulation, 14(3), 579–587. https://doi.org/10.1016/j.brs.2021.03.011
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., & Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261. https://doi.org/10.1038/nature07868
Laurienti, P. J., Perrault, T. J., Stanford, T. R., Wallace, M. T., & Stein, B. E. (2005). On the use of superadditivity as a metric for characterizing multisensory integration in functional neuroimaging studies. Experimental Brain Research, 166(3), 289–297. https://doi.org/10.1007/s00221-005-2370-2
Lenc, T., Keller, P. E., Varlet, M., & Nozaradan, S. (2018). Neural tracking of the musical beat is enhanced by low-frequency sounds. Proceedings of the National Academy of Sciences, 115(32), 8221–8226. https://doi.org/10.1073/pnas.1801421115
Metzger, B. A., Magnotti, J. F., Wang, Z., Nesbitt, E., Karas, P. J., Yoshor, D., & Beauchamp, M. S. (2020). Responses to Visual Speech in Human Posterior Superior Temporal Gyrus Examined with iEEG Deconvolution. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 40(36), 6938–6948. https://doi.org/10.1523/JNEUROSCI.0279-20.2020
Moreau, C. N., Joanisse, M. F., Mulgrew, J., & Batterink, L. J. (2022). No statistical learning advantage in children over adults: Evidence from behaviour and neural entrainment. Developmental Cognitive Neuroscience, 57, 101154. https://doi.org/10.1016/j.dcn.2022.101154
Nozaradan, S., Peretz, I., & Mouraux, A. (2012). Selective Neuronal Entrainment to the Beat and Meter Embedded in a Musical Rhythm. Journal of Neuroscience, 32(49), 17572–17581. https://doi.org/10.1523/JNEUROSCI.3203-12.2012
Obleser, J., & Kayser, C. (2019). Neural Entrainment and Attentional Selection in the Listening Brain. Trends in Cognitive Sciences, 23(11), 913–926. https://doi.org/10.1016/j.tics.2019.08.004
Peter, V., Goswami, U., Burnham, D., & Kalashnikova, M. (2023). Impaired neural entrainment to low frequency amplitude modulations in English-speaking children with dyslexia or dyslexia and DLD. Brain and Language, 236, 105217. https://doi.org/10.1016/j.bandl.2022.105217
Shen, L., Lu, X., Wang, Y., & Jiang, Y. (2023). Audiovisual correspondence facilitates the visual search for biological motion. Psychonomic Bulletin & Review, 30(6), 2272–2281. https://doi.org/10.3758/s13423-023-02308-z
Shen, L., Lu, X., Yuan, X., Hu, R., Wang, Y., & Jiang, Y. (2023). Cortical encoding of rhythmic kinematic structures in biological motion. NeuroImage, 268, 119893. https://doi.org/10.1016/j.neuroimage.2023.119893
Simion, F., Regolin, L., & Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences, 105(2), 809–813. https://doi.org/10.1073/pnas.0707021105
Stanford, T. R., Quessy, S., & Stein, B. E. (2005). Evaluating the Operations Underlying Multisensory Integration in the Cat Superior Colliculus. Journal of Neuroscience, 25(28), 6499–6508. https://doi.org/10.1523/JNEUROSCI.5095-04.2005
Stevenson, R. A., Ghose, D., Fister, J. K., Sarko, D. K., Altieri, N. A., Nidiffer, A. R., Kurela, L. R., Siemann, J. K., James, T. W., & Wallace, M. T. (2014). Identifying and Quantifying Multisensory Integration: A Tutorial Review. Brain Topography, 27(6), 707–730. https://doi.org/10.1007/s10548-014-0365-7
Troje, N. F., & Westhoff, C. (2006). The Inversion Effect in Biological Motion Perception: Evidence for a “Life Detector”? Current Biology, 16(8), 821–824. https://doi.org/10.1016/j.cub.2006.03.022
Ujiie, Y., Asai, T., & Wakabayashi, A. (2015). The relationship between level of autistic traits and local bias in the context of the McGurk effect. Frontiers in Psychology, 6. https://doi.org/10.3389/fpsyg.2015.00891
Vallortigara, G., & Regolin, L. (2006). Gravity bias in the interpretation of biological motion by inexperienced chicks. Current Biology, 16(8), R279–R280. https://doi.org/10.1016/j.cub.2006.03.052
Vanrie, J., & Verfaillie, K. (2004). Perception of biological motion: A stimulus set of human point-light actions. Behavior Research Methods, Instruments, & Computers, 36(4), 625–629. https://doi.org/10.3758/BF03206542
Wang, L., & Jiang, Y. (2012). Life motion signals lengthen perceived temporal duration. Proceedings of the National Academy of Sciences of the United States of America, 109(11), E673-677. https://doi.org/10.1073/pnas.1115515109
Wang, L., Yang, X., Shi, J., & Jiang, Y. (2014). The feet have it: Local biological motion cues trigger reflexive attentional orienting in the brain. NeuroImage, 84, 217–224. https://doi.org/10.1016/j.neuroimage.2013.08.041
Wang, Y., Zhang, X., Wang, C., Huang, W., Xu, Q., Liu, D., Zhou, W., Chen, S., & Jiang, Y. (2022). Modulation of biological motion perception in humans by gravity. Nature Communications, 13(1), Article 1. https://doi.org/10.1038/s41467-022-30347-y
Wright, T. M., Pelphrey, K. A., Allison, T., McKeown, M. J., & McCarthy, G. (2003). Polysensory Interactions along Lateral Temporal Regions Evoked by Audiovisual Speech. Cerebral Cortex, 13(10), 1034–1043. https://doi.org/10.1093/cercor/13.10.1034





