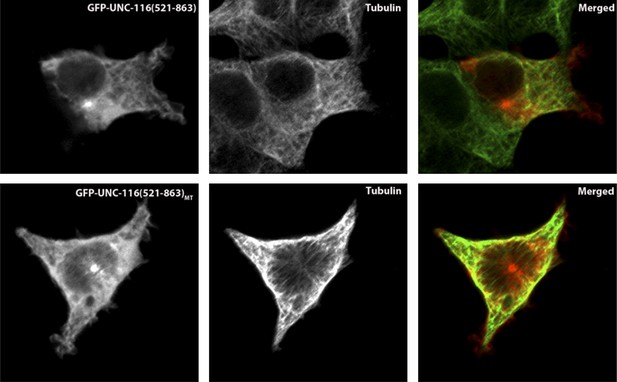
Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans
Figures
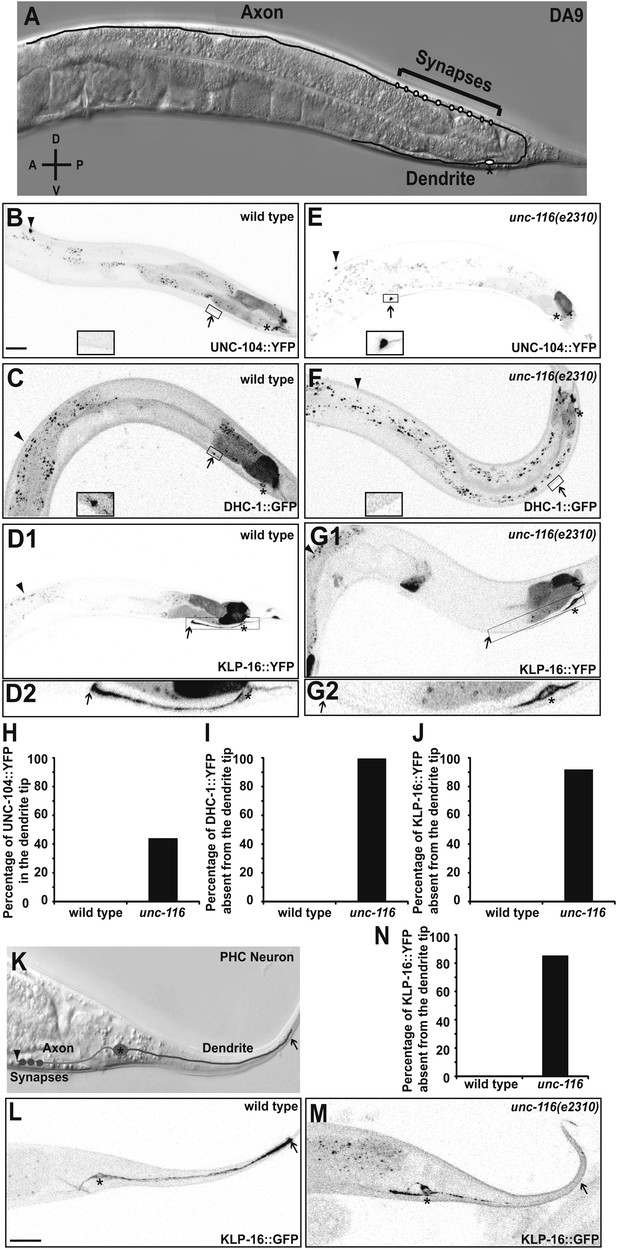
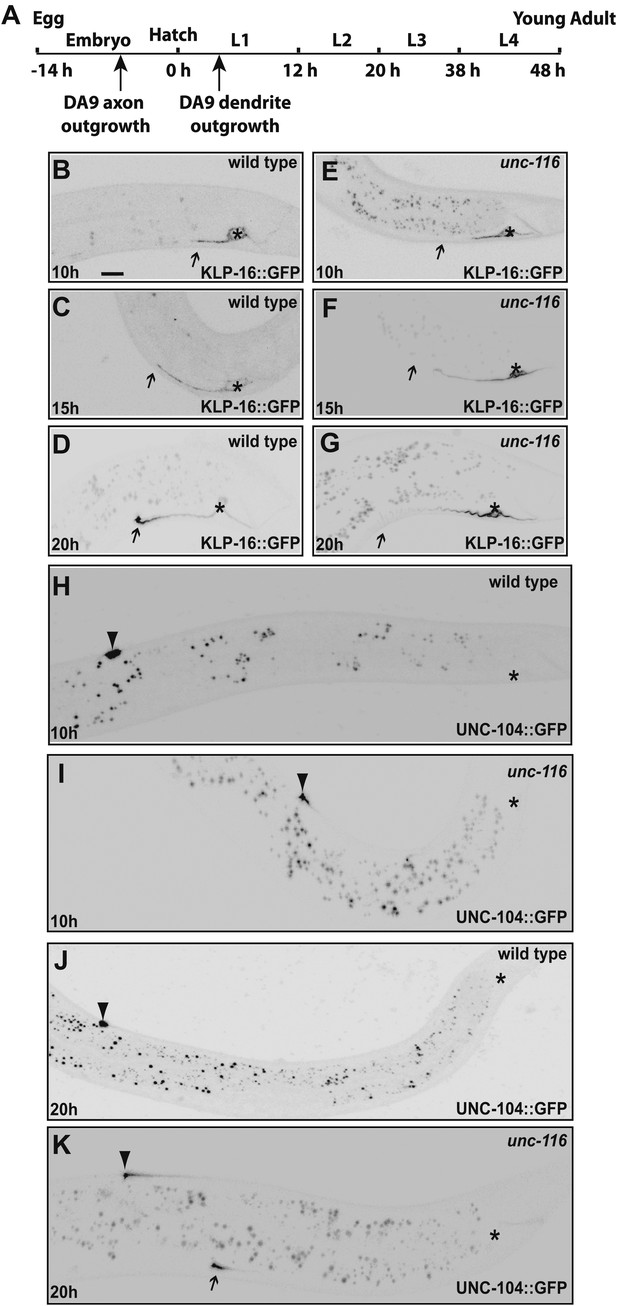
UNC-116 (kinesin-1) is required for the minus-end-out MT polarity in the DA9 dendrite.
(A) Schematic diagram of the morphology of the DA9 neuron. Asterisk denotes DA9 or PHC cell body (throughout all images); D: dorsal; V: ventral; A: anterior; P: posterior. (B) and (E) Localization of UNC-104::YFP in a representative wild-type (B) or unc-116 worm (E). Note that UNC-104::YFP is enriched in the axonal tip (denoted by an arrowhead throughout) in wild-type, while it is enriched at the tips of both the axon and dendrite (dendritic tip is marked by an arrow, dashed black box and shown in higher magnification micrographs throughout) in unc-116 animals. (C) and (F) Localization of DHC-1::GFP in a representative wild-type (C) or unc-116 worm (F). (D) and (G) Localization of KLP-16::YFP in a representative wild-type (D1) or unc-116 worm (G1). The dendrite is shown in higher magnification for a wild-type (D2) or unc-116 animals (G2). (H)–(J) Quantification of fractions of worms with qualitative defects in UNC-104::YFP (H), DHC-1::GFP (I), and KLP-16::YFP(J) localization in DA9 dendrite (n > 50 for each genotype). (K) Schematic diagram of the morphology of the PHC neuron. (L) and (M) Localization of KLP-16::YFP in a representative wild-type (L) or unc-116 worm (M). (N) Quantification of fractions of worms with qualitative defects in KLP-16::YFPlocalization in the PHC dendrite (n > 50 for each genotype). The scale bar represents 10 μm.
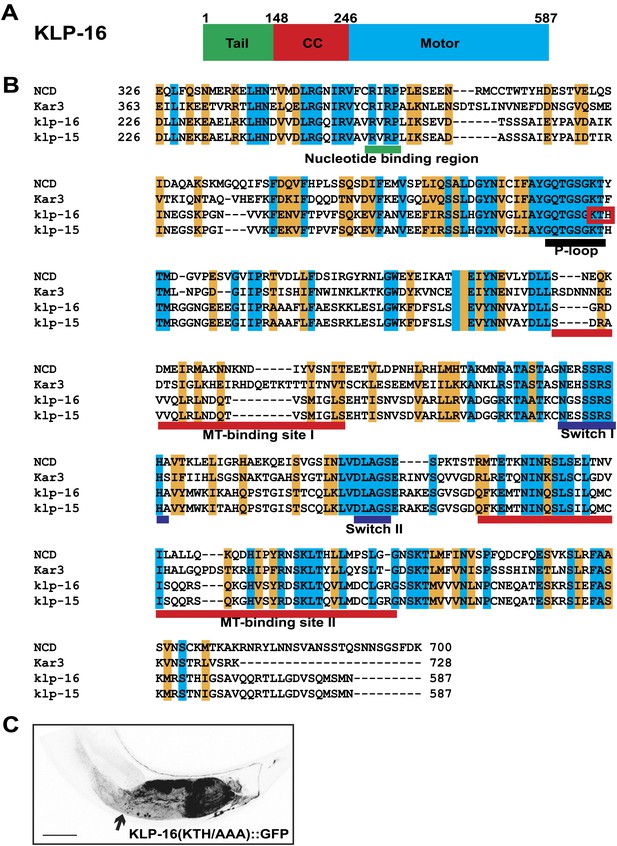
Kinesin motor activity is required for the enrichment of KLP-16::YFP in dendrite.
(A) Domain structure of KLP-16. From N to C terminus, tail domain is in green, coiled-coil region is in red, and motor domain is in blue. The numbers of amino acids are on top. (B) Amino acid sequence alignment of KLP-16 motor domain with KLP-15, NCD, and Kar3 proteins. Identical residues are labeled in blue and similar residues are labeled in orange. Functional regions within the motor domain are underlined. (C) A representative image of KLP-16 (KTH/AAA)::GFP expressed in DA9 neuron. The scale bar represents 10 μm.
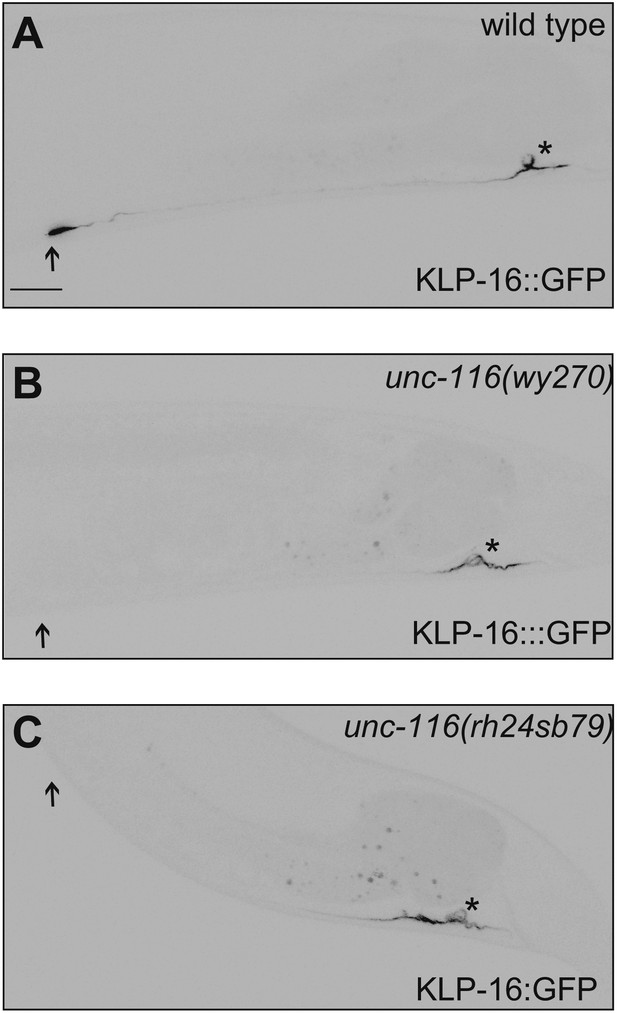
DA9 dendrite MT polarity is altered in unc-116 mutants.
(A)–(C) Representative images showing KLP-16::GFP localization in wild-type (A), unc-116 (wy270) (B), and unc-116 (rh24 sb79) animals (C). Asterisk indicates cell body; arrowhead indicates the tip of DA9 dendrite. The scale bar represents 10 μm.
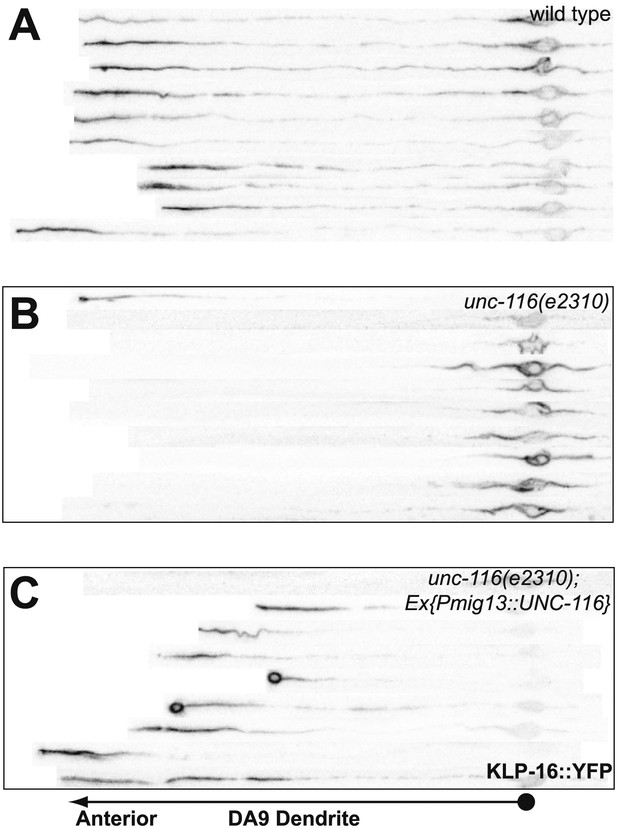
UNC-116 acts cell autonomously in DA9 neuron.
(A)–(C) Confocal micrographs of 10 wild-type (A), unc-116 (e2310) (B), and unc-116;Ex[Pmig13::unc-116] DA9 ventral axon, cell body and dendrite, straightened and aligned along their A-P axis.
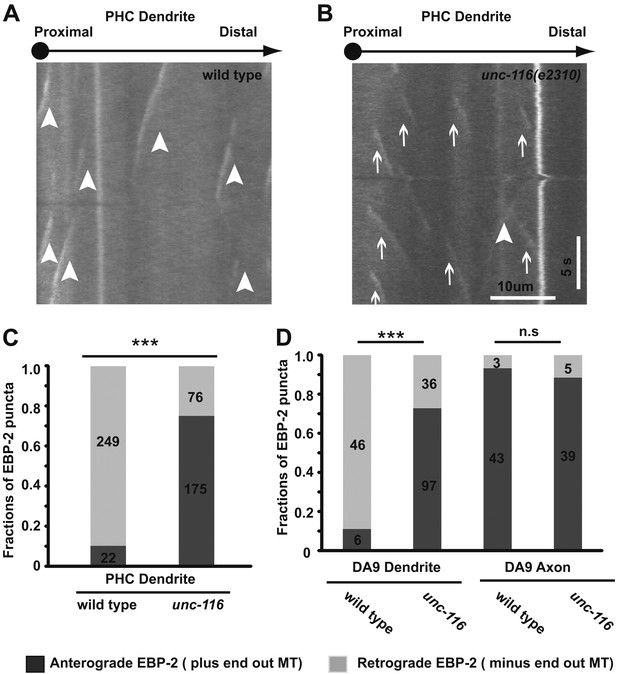
Dendrite MTs are plus-end out in the unc-116(e2310) mutant.
(A) and (B) Representative kymographs of moving EBP-2::GFP puncta in the PHC neuron dendrite of wild-type (A) and unc-116 animals (B). The cell body is to the left in both panels. Time runs top to bottom; arrowheads mark retrogradely moving puncta; arrows mark anterogradely moving puncta. (C) and (D) Bar graphs of the fraction of anterograde and retrograde movements in PHC (C) and DA9 neurons (D). MT, microtubule; numbers within each column denote the number of puncta counted in the corresponding categories; ***p<0.001, χ2 test.
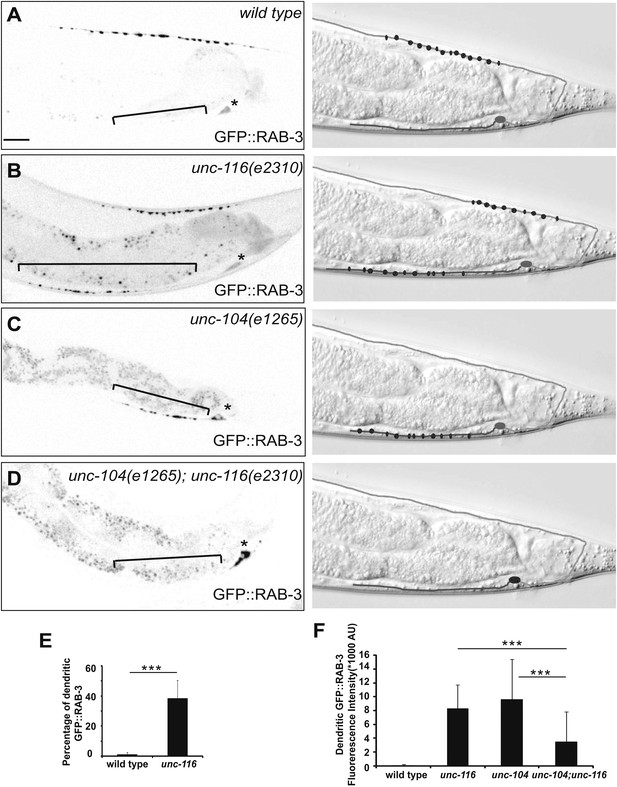
Dendrite exhibits axonal-like properties in unc-116 mutants.
(A)–(D) Distribution of GFP::RAB-3 puncta (left panels) and represented schematic diagrams (right panels) in wild-type (A), unc-116 (B), unc-104 (C), and unc-116;unc-104 mutant animals (D). (E) Average percentage of GFP::RAB-3 fluorescence intensity in the dendrite (n = 20). (F) Quantification of GFP::RAB-1 fluorescence intensity in the dendrite (n = 20). ***p<0.0001. Student's t-test. The scale bar represents 10 μm. Asterisk denotes DA9 cell body.
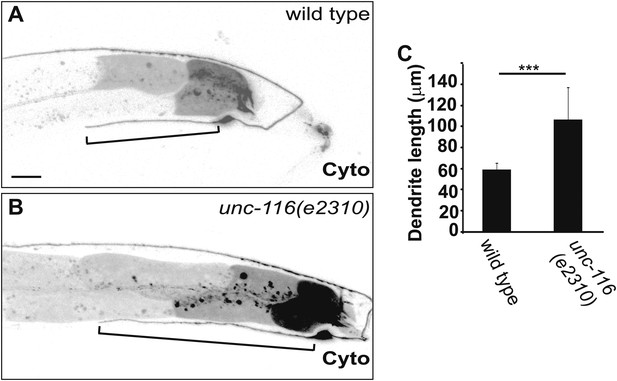
DA9 dendrite is longer in unc-116 mutants.
(A) and (B) Micrographs of representative wild-type (A) and unc-116 (e2310) (B) adults expressing cytoplasmic mCherry in DA9. (C) Average length of DA9 dendrite in 20 wild-type and unc-116 (e2310) adults. The scale bar represents 10 μm. Bracket indicates dendrite; error bars indicate standard deviations. ***p<0.0001. χ2 test.
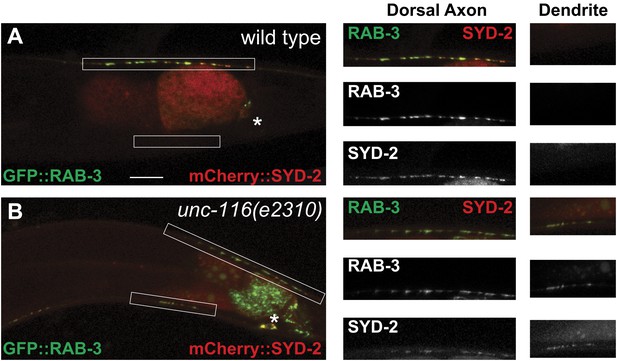
Presynaptic proteins are mis-localized in unc-116 mutant.
(A) and (B) Distribution of synaptic vesicle marker GFP::RAB-3 and an active zone marker mCherry::SYD-2 in wild-type and unc-116 (e2310) mutant. The scale bar represents 10 μm. The high magnifications are shown in the right panels.
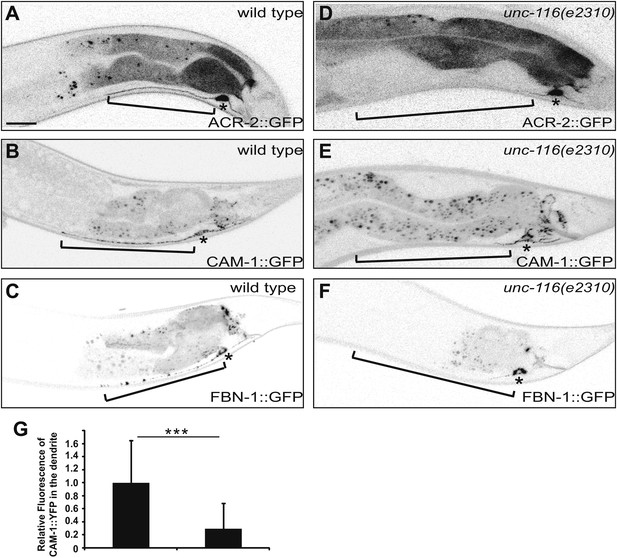
DA9 dendrite fails to accumulate dendritic proteins in unc-116 mutants. (A) and (D) Distribution of the postsynaptic neurotransmitter receptor ACR-2::GFP in wild-type (A) and unc-116 (D) animals. Bracket indicates dendrite region. (B) and (E) Localization of CAM-1::YFP in a representative wild-type (B) or unc-116 worm (E). (C) and (F) Localization of FBN-1::YFP in a representative wild-type (C) or unc-116 worm (F). (G) Quantification of CAM-1::YFP fluorescence intensity in the dendrite (n = 20). ***p<0.0001. Student's t-test. The scale bar represents 10 μm. Bracket indicates dendrite. Asterisk denotes DA9 cell body.
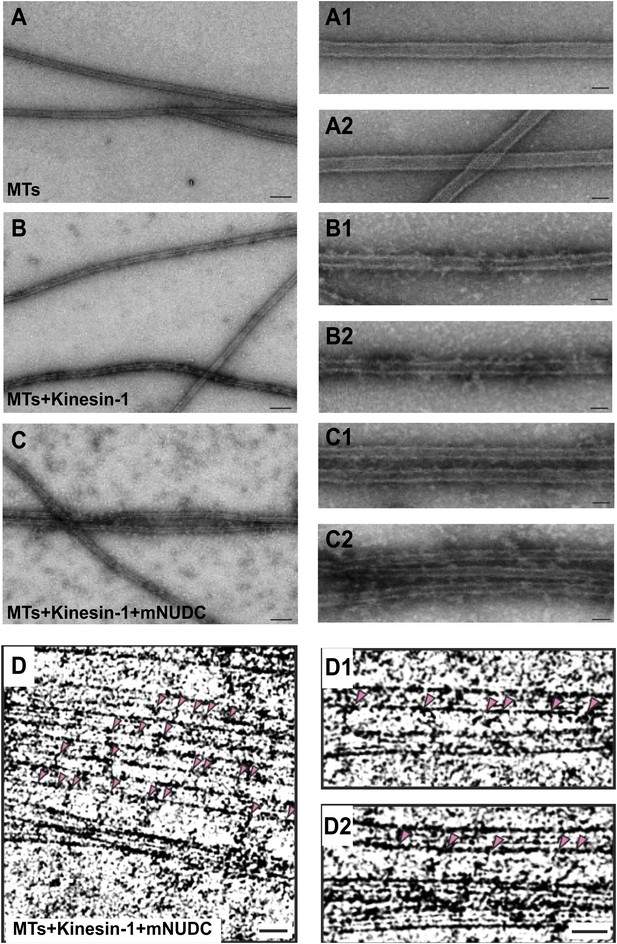
Purified kinesin-1 complex bundles anti-parallel MTs in vitro.
(A)–(C) Negatively stained EM images of (A) MTs alone, (B) kinesin-1-MTs, and (C) kinesin-1-NudC-MTs. Two enlarged images are in right row. Scar bar is 100 nm from (A–C), and 30 nm in the enlarged images. Note that long spanned MT bundles were observed only in (C) when the samples mixed with both Kinesin-1 and NudC. Deeply stained cross-bridging molecules are seen between adjacent MTs.Cryo-EM images of microtubules of (D) kinesin-NudC microtubles, (D1) microtubules alone and (D2) kinesin-microtubules.
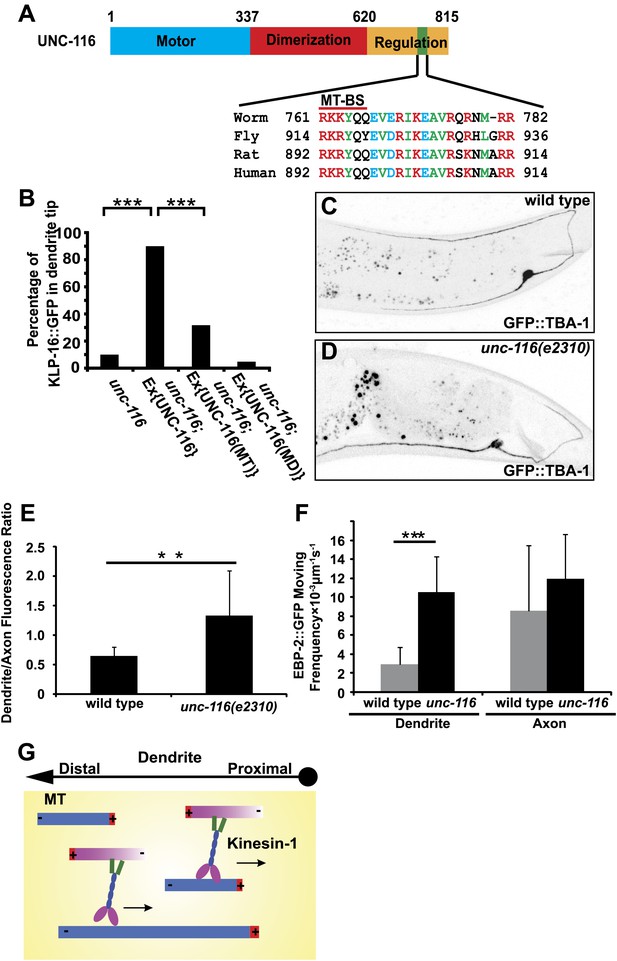
UNC-116/kinesin-1 orients dendritic MTs.
(A) Domain structure of UNC-116 protein with motor domain in blue, dimerization region in red, and C-terminal regulation domain in orange, length of amino acid is shown. (B) Cell-autonomous rescue of KLP-16::YFP localization. MD, mutant swapping the amino acids ‘KTH' in P-loop of UNC-116 motor domain with ‘AAA'; MT, mutant swapping the MT-BS in UNC-116 tail region with ‘AAAYAA'. n > 50 per group. Error bar indicates standard deviation; ***p<0.0001, χ2 test. (C) and (D) MTs structure highlighted by GFP::TBA-1 in a representative wild-type (C) or unc-116 worm (D). Quantification of GFP::TBA-1 fluorescence density ratio between the dendrite and the ventral axon in wild-type and the unc-116(e2310) animals. (E) Quantification of EBP-2::GFP puncta moving frequency in the dendrite of wild type and the unc-116(e2310) animals. n > 20 per group. Error bar indicates standard deviation; **p<0.001, ***p<0.0001, Student's t-test. (F) A schematic model showing kinesin-1 cross-linking and sliding MTs out of the dendrite.
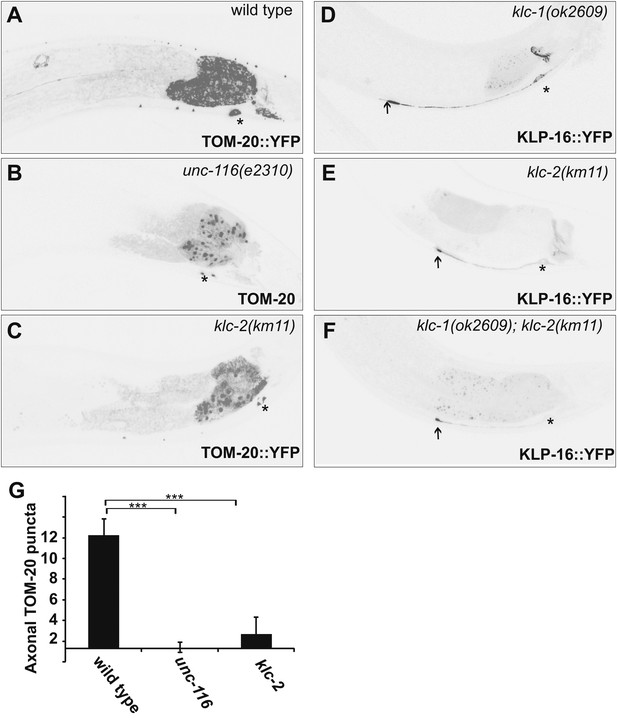
Kinesin light chains are not required for dendrite MT polarity.
(A)–(C) Distribution of a mitochondria marker, TOM-20::YFP in a representative wild-type (A), unc-116 (e2310) (B), or klc-2 (km11) (C) worm. (D)–(F) Localization of KLP-16::YFP in a representative wild-type (D) , klc-1 (ok2609) (E), or klc-2 (km11) worm (F). (G) Quantification of axonal TOM-20::YFP puncta number in wild-type, unc-116 (e2310), or klc-2 (km11) worms (n = 20). The scale bar represents 10 μm. Error bars indicate standard deviations. ***p<0.0001. χ2 test.
Videos
Movement of EBP-2::GFP puncta in the ventral axon of wild-type DA9 neuron. Cell body is to the left. Displayed 10× speed.
Movement of EBP-2::GFP puncta in the dendite of wild-type DA9 neuron. Cell body is to the left. Displayed 10× speed.
Movement of EBP-2::GFP puncta in the dendrite of wild-type PHC neuron. Cell body is to the left. Displayed 10× speed.
Movement of EBP-2::GFP puncta in the ventral axon of unc-116 (e2310) DA9 neuron. Cell body is to the left. Displayed 10× speed.
Movement of EBP-2::GFP puncta in the dendrite of unc-116 (e2310) DA9 neuron. Cell body is to the left. Displayed 10× speed.