Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract
Figures
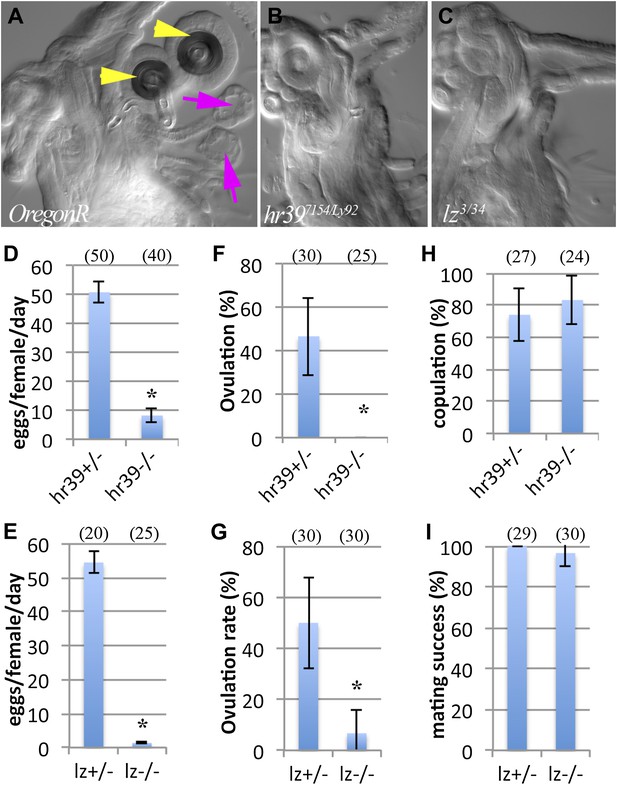
Female reproductive glands are essential for ovulation.
(A)–(C) DIC images of Oregon-R (A), Hr397154/Ly92 (B) and lz3/34 (C) mutant female lower reproductive tracts. Both spermathecae (yellow arrowheads) and parovaria (magenta arrows) are absent in the mutant animals. Bar graphs display the rate of egg laying (D and E), ovulation frequency (F and G), and copulation frequency (H and I) for the two mutant genotypes, and heterozygous controls. In all figures, the number of egg laying groups or mating pairs is shown in brackets. Error bars are SEM, or 95% confidence intervals. *p<0.01 (Fisher's exact test, or Student t-test).
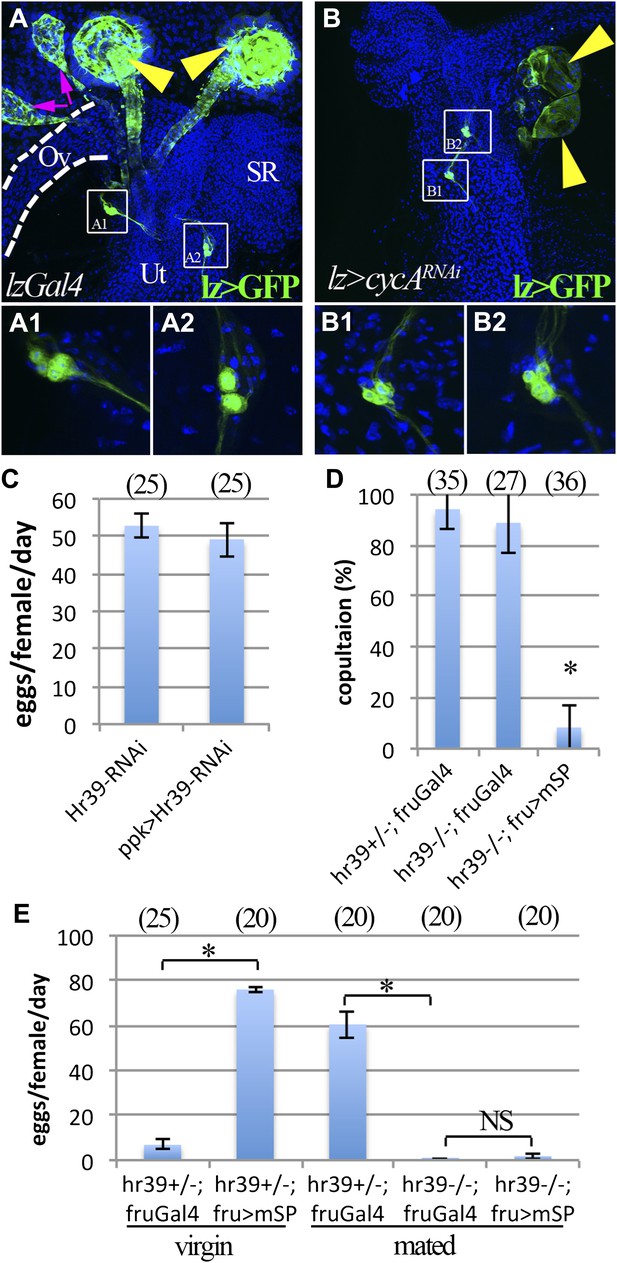
lz and Hr39 are not required in reproductive tract neurons.
(A) lz expression (lzGal4 driving UAS-mCD8::GFP) in control female reproductive tract. Spermathecae (yellow arrowheads); parovaria (magenta arrowheads). Ov: Ovuduct; SR: Seminal receptacle; Ut: Uterus. Two sets of lz+ sensory neurons are illustrated at higher magnification in (A1 and A2). (B) lz expression in female reproductive tract expressing lzGal4>UAScycA (lz>cycARNAi). lz+ sensory neurons are not affected (B1 and B2). (C) Egg production is not affected by expressing Hr39-RNAi in ppk+ neurons of the reproductive tract. (D) Ectopic expression of mSP in fru+ reproductive tract neurons reduces virgin female copulation rate, even when neurons are mutant for Hr39. (E) Ectopic mSP in fru+ neurons is sufficient to induce egg laying in control virgin females but not in Hr39−/− females even in the presence of males. * indicates p<0.01 and NS indicates p>0.05.
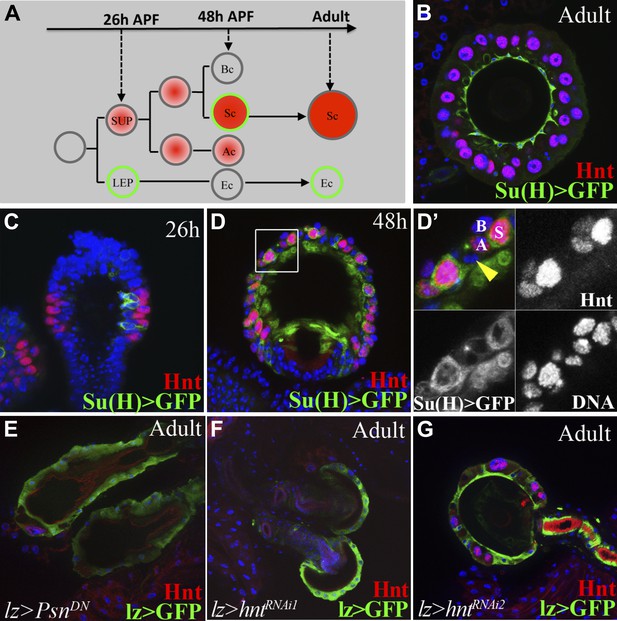
Notch signaling and Hindsight are required to form reproductive gland secretory cells.
(A) The cell lineage underlying secretory cell development (Sun and Spradling, 2012). Notch signaling activity (green); Hnt expression (red). Ac: Apical cell; Bc: Basal cell; LEP: Lumen epithelial precursor; Sc: Secretory cell; SUP: Secretory unit precursor. (B)–(D) Notch activity (green) and Hnt (red) in spermathecae of adults (B), 26 hr pupae (APF) (C), and 48 hr APF (D). (D') shows the boxed region from (D). Yellow arrowhead: Epithelial cell. (E)–(G) Adult spermathecae from females expressing lz>Psn[DN] (E) or lz>hntRNAi (F–G) during gland development. lz (green) marks epithelial cells; Hnt (red) marks secretory cells.
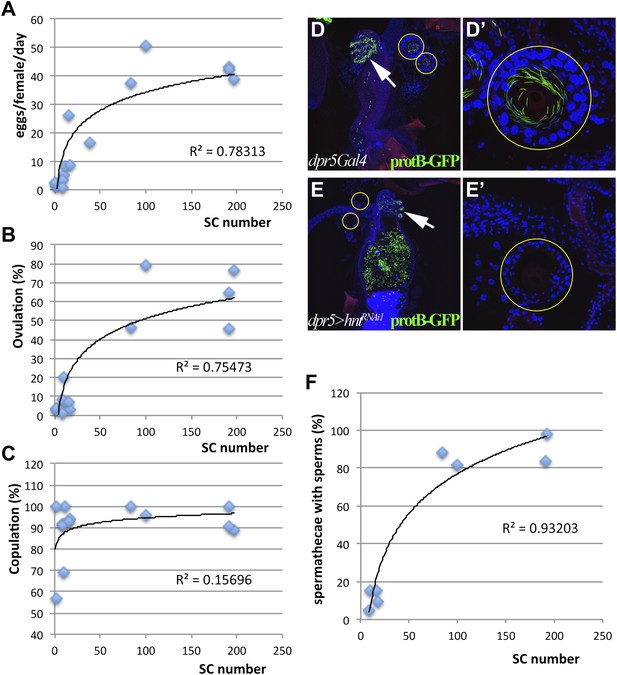
Female reproductive tract secretory cells mediate ovulation and sperm storage.
(A)–(C) Relationship between secretory cell (SC) number and egg laying rate (A); percent ovulation (B); or percent copulation (C). Pooled data from genotypes in Table 1. Female reproductive tracts (D and E) and spermathecae (yellow circles in D and E; shown at higher magnification: D' and E') from normal females (dpr5Gal4 alone) (D) or females lacking SCs (dpr5Gal4>hntRNAi) (E) 6 hr after mating to males whose sperm nuclei are marked with protB-GFP (green). Seminal receptacle (white arrow). (F) Relationship between secretory cell number and the percentage of spermathecae with >5 sperm. Pooled data from genotypes in Table 1.
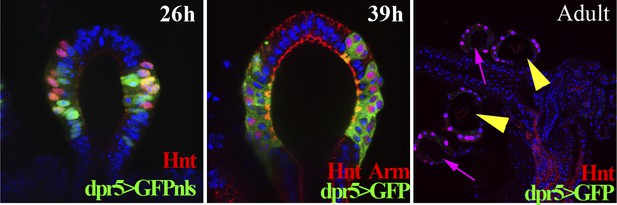
The expression pattern of the dpr5Gal4 line in spermathecae at 26 hr (using UAS-GFPnls), 39 hr APF (using UAS-GFP) and in the adult female lower reproductive tract (using UAS-GFP).
https://doi.org/10.7554/eLife.00415.008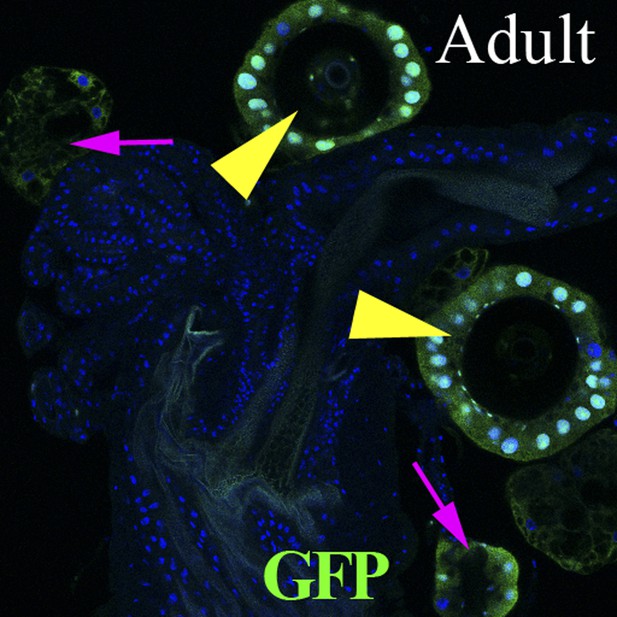
Lineage-marked progeny of dpr5+ cells (green) in the female reproductive tract, showing labeling of SC cells.
Spermathecae (yellow arrowheads); parovaria (magenta arrows).
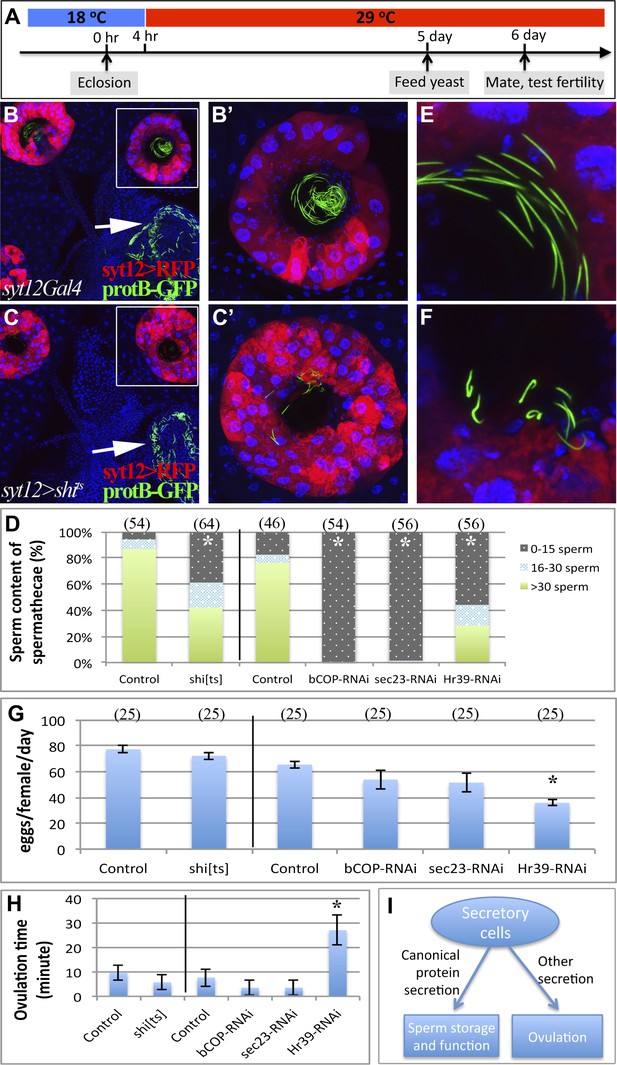
Canonical protein secretion from glandular secretory cells is required for sperm storage but not for ovulation.
(A) Experimental scheme for testing adult secretory cell function using temperature sensitive shits or GAL80ts. (B) and (C) Dynamin (Shi) is required for sperm storage. Female reproductive tract of syt12Gal4 control (B) or syt12Gal4 driving shits expression (C) 6 hr after mating to protB-GFP males at 29°C. syt12Gal4 expression is restricted to secretory cells as showed by UAS-RFP (red). (B') and (C'): Higher magnification of boxed spermathecae; seminal receptacle contain sperm (white arrows). (D) Sperm content of spermathecae (three classes) is reduced in flies with indicated genotype (x axis) at 29°C. Bracket: Number analyzed. *p<0.01 (chi-square test). (E) and (F) Abnormal morphology of spermathecal sperm in shits females at 29°C (F) compared to control (E). Egg laying rate (G) and ovulation time (from Table 2) (H) in flies with the indicated genotypes (x axes). *p<0.05 (Students t-test or Fisher's exact test). (I) Secretory cells use distinct secretory pathways to control sperm storage and ovulation.
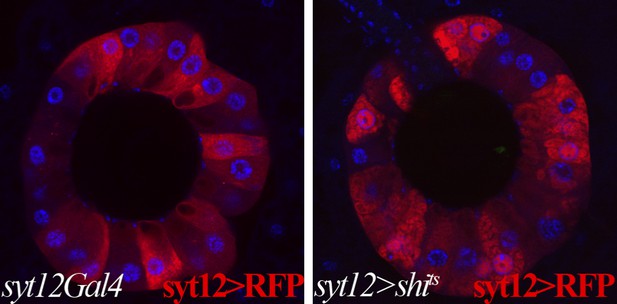
Membrane trafficking defects are observed in SCs when protein secretion is disrupted.
Control secretory cells (left): syt12Gal4 > UAS-RFP; shits-expressing secretory cells (right): syt12Gal4 > UAS-RFP UAS-shits. RFP foci are visible in shits-expressing SCs but not in controls. Single confocal sections are shown.
Tables
The effect of altering secretory cell (SC) number in female reproductive glands on egg laying, ovulation, copulation, and sperm storage in spermathecae
| Genotype | Female glands | Egg laying in 2 days | Ovulation in 6 hr | Copulation in 6 hr | Sperm storage in 6 hr | |||||
| N | SC/Female (Avg. ± SD) | N | Eggs/female/Day (Avg. ± SEM) | N | Ovulation (%) | N | Copulation (%) | N | Spermathecae with sperm (%) | |
| lzGal4 | 10 | 197.0 ± 18.0 | 45 | 38.9 ± 3.9 | 30 | 76.7 | 18 | 89.0 | ||
| lz>cycARNAi | 36 | 2.0 ± 2.6* | 25 | 1.0 ± 1.0* | 30 | 3.3* | 23 | 56.5 | ||
| lz>hr39RNAi | 15 | 10.4 ± 7.4* | 25 | 5.2 ± 0.8* | 30 | 20* | 13 | 69.2 | ||
| lz>hntRNAi1 | 23 | 11.2 ± 8.4* | 45 | 8.0 ± 1.9* | 30 | 6.7* | 27 | 100.0 | ||
| lz>hntRNAi2 | 22 | 39.4 ± 12.1* | 25 | 16.5 ± 1.5† | ||||||
| lz>PsnDN | 25 | 1.8 ± 1.9* | 25 | 2.0 ± 1.8* | 25 | 4* | 25 | 100.0 | ||
| lz>Su(H)DN | 16 | 0.9 ± 1.0* | 15 | 2.5 ± 2.0* | ||||||
| dpr5Gal4 | 5 | 192.0 ± 15.4 | 40 | 43.0 ± 4.5 | 31 | 64.5 | 25 | 100.0 | 50 | 98.0 |
| dpr5>cycARNAi | 25 | 9.3 ± 3.7* | 25 | 3.3 ± 1.5* | 35 | 8.6* | 35 | 91.4 | 65 | 15.4* |
| dpr5>hr39RNAi | 10 | 191.3 ± 15.3 | 15 | 42.1 ± 2.6 | 24 | 45.8 | 21 | 90.5 | 37 | 83.8 |
| dpr5>hntRNAi1 | 18 | 17.1 ± 6.3* | 50 | 8.4 ± 1.8* | 34 | 2.9* | 34 | 94.1 | 64 | 9.4* |
| dpr5>hntRNAi2 | 13 | 99.5 ± 20.6* | 25 | 50.4 ± 2.8 | 24 | 79.2 | 24 | 95.8 | 49 | 81.6 |
| dpr5>NRNAi | 25 | 9.0 ± 3.2* | 25 | 0.9 ± 0.6* | 25 | 1* | 23 | 91.3 | 42 | 4.8* |
| dpr5>PsnDN | 16 | 83.9 ± 11.5* | 25 | 37.4 ± 7.5 | 26 | 46.2 | 26 | 100.0 | 52 | 88.5 |
| dpr5>Su(H)DN | 20 | 16.1 ± 4.5* | 25 | 26.2 ± 1.7† | 14 | 7.1* | 14 | 92.9 | 26 | 15.4* |
-
*
p<0.001. T-test was used for secretory cell number and egg laying. Fisher's exact test was used for ovulation, copulation, and sperm storage.
-
†
p<0.01.
The effect of disrupting protein secretion or Hr39 expression during adulthood on the rate of egg laying and uterine egg content
| Genotype | Egg laying in 2 days* | Egg distribution in 6 hr | Egg laying time (min) | ||||
| N | Eggs/female/day | N | Uterus with egg (%) | Total time | Ovulation time | Uterus time | |
| syt12Gal4 | 25 | 77.3 ± 2.3 | 28 | 42.9 ± 18.3 | 17.1 ± 0.5 | 9.8 ± 3.1 | 7.3 ± 3.1 |
| syt12>shits | 25 | 72.2 ± 2.4 | 32 | 68.8 ± 16.1 | 18.3 ± 0.6 | 5.7 ± 2.9 | 12.6 ± 3.0 |
| syt12Gal4 | 25 | 65.4 ± 2.3 | 29 | 62.1 ± 17.7 | 20.2 ± 0.7 | 7.6 ± 3.6 | 12.5 ± 3.6 |
| syt12>βCOPRNAi | 25 | 53.8 ± 6.3 | 28 | 85.7 ± 13.0 | 24.5 ± 2.9† | 3.5 ± 3.2 | 21.0 ± 4.0† |
| syt12>sec23RNAi | 25 | 51.5 ± 6.2 | 29 | 86.2 ± 12.6 | 25.7 ± 3.1† | 3.5 ± 3.3 | 22.1 ± 4.2† |
| syt12>Hr39RNAi | 25 | 35.8 ± 2.2† | 30 | 26.7 ± 15.8† | 36.9 ± 2.3† | 27 ± 6.1† | 9.8 ± 5.9 |
-
*
1 day = 22 hr at 29°C.
-
†
p<0.05. All data are mean ± 95% confidence interval. T-test was used for egg laying, while Fisher's exact test was used for egg distribution.





