Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage
Figures
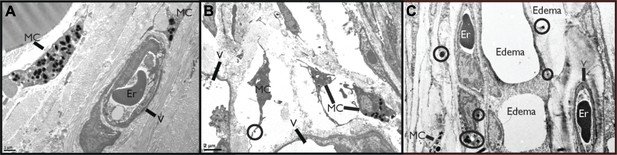
DENV-Induced MC activation and microstructural changes surrounding blood vessels.
TEMs acquired from mouse ear tissue 24 hr after either saline injection (A) or 1 × 105 PFU of DENV (B) and (C). Figure labels for (A)–(C): MC: mast cell; V: vessel; Er: erythrocyte. (A) In control tissues, apparently quiescent, granulated, MCs can be visualized in proximity to a blood vessel. (B) A field containing many MCs that appear activated due to their reduced granularity and cytoplasmic projections that are characteristic of MC degranulation. A granule that is being released is circled. This image also contains portions of two vessels, visible on the left and bottom sides. (C) MCs are closely associated with vessels in tissue that shows signs of fluid pooling or edema. Extracellular granules are visible throughout the tissue and are circled. A blood vessel containing an erythrocyte is labeled on the right side of the image and a second vessel, likely a lymphatic vessel, is visible on the left side.
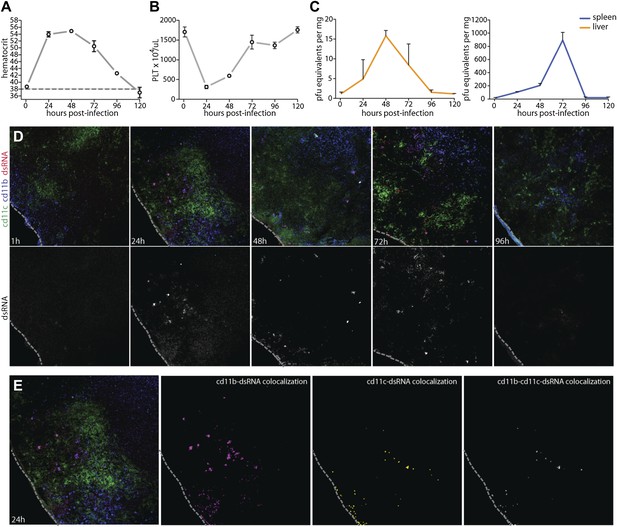
Vascular pathology and DENV replication in the WT mouse model.
Cohorts of mice were monitored for 5 days following the experimental establishment of systemic infection using DENV clinical isolate Eden2, by intra-peritoneally. injection. Blood was obtained at 1 hr after infection and at subsequent 24 hr time points then analyzed to determine the (A) hematocrit values and (B) platelet concentration for individual infected mice (n = 3–4 at each time point). A dashed line represents the average baseline values for uninfected, naïve animals. (C) At the same time points, tissue was harvested from the spleen and liver and real time PCR was performed after cDNA conversion in order to quantitate DENV genome copies (PFU equivalents) in the tissue, which was normalized to the tissue mass. Error bars for panels (A–C) represent the SEM and are included for all groups; where they are not visible, variation was small. (D) Images of spleen sections along this time course are presented, where 10-μm-thick sections were stained for DENV replication (dsRNA, red), monocytes and/or macrophages (cd11b, blue), and dendritic cells (cd11c, green). Bottom panels in (D) show the isolated dsRNA panel. (E) Co-localization images were generated using ImageJ software and reveal that dsRNA staining co-localized predominantly with the monocyte/macrophage marker cd11b, and to a lesser extent, the DC marker, cd11c. For panels (D and E), the dashed line denotes the border of the spleen in the tissue section.
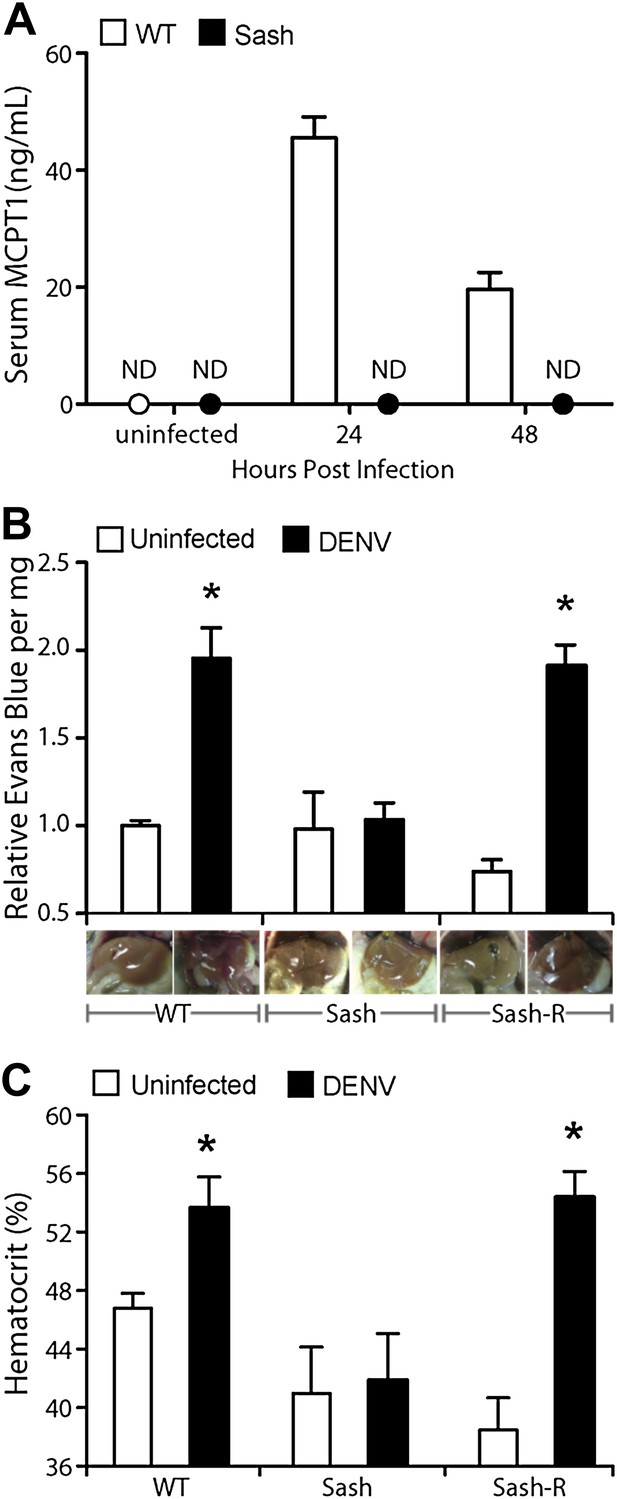
Vascular leakage during DENV infection is MC-dependent.
(A) Graph depicts the serum concentration of MCPT1, which was quantified using serum obtained from WT or Sash mice, 24 and 48 hr after intra-peritoneally. injection with 1 × 106 PFU of DENV. MCPT1 was not detected (ND) in uninfected WT mice and uninfected or infected Sash mice. Error bars represent the SEM of ELISA replicates using pooled serum samples from n = 4 animals. To compare vascular leakage in infected vs uninfected WT, Sash, and Sash-R mice, (B) hematocrit analysis using heparinized blood and (C) quantitation of Evans blue dye leakage into liver tissue were performed 24 hr after infection with DENV. Representative images of mouse livers after saline perfusion are presented below the respective data bars to support that visually perceivable vascular leakage occurred in DENV-infected animals. For (B and C), error bars represent the SEM where values were obtained from individual infected mice n = 3–6 per group. * indicates a significant increase over uninfected controls; p≤0.05.
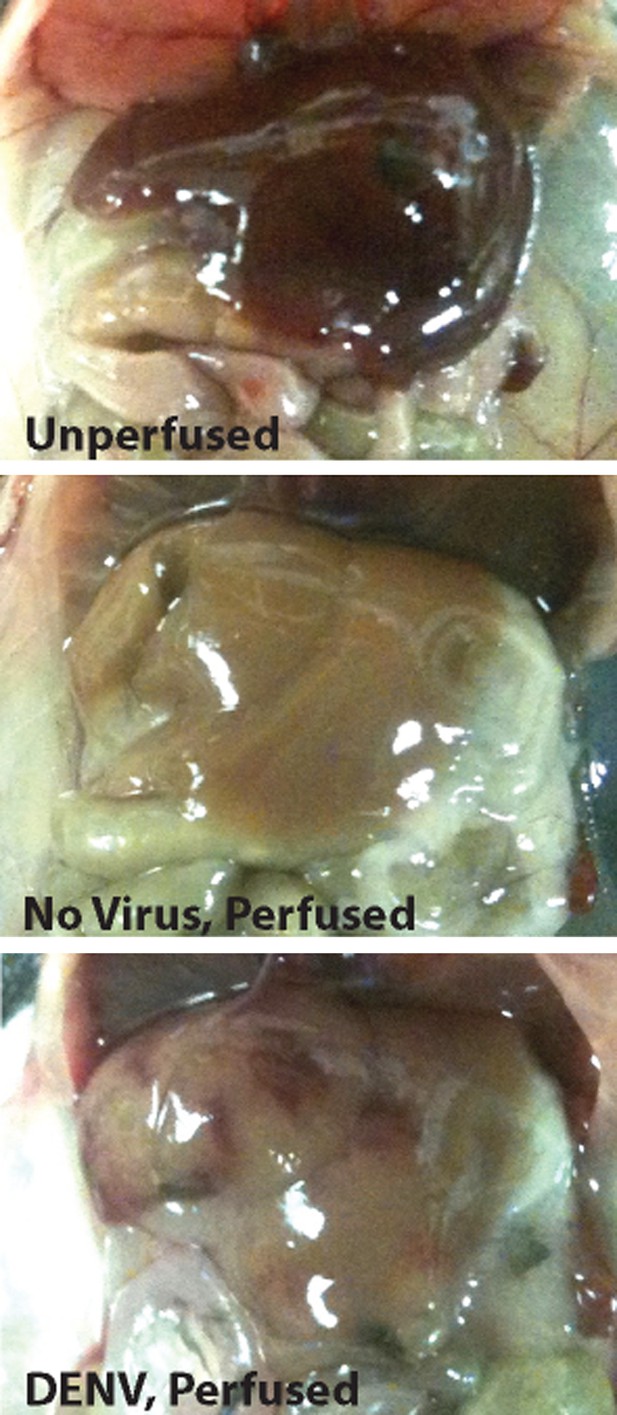
Representative images of livers from uninfected mice, or mice infected with 1 × 106 PFU of DENV.
Livers appeared normal during necropsy at 24 hr following injection with Evans blue dye. Perfusion of mice with saline eliminates blood in the vasculature. In mice infected with DENV, vascular permeability can be visualized due to remaining blood and Evans blue dye within tissues after saline perfusion.
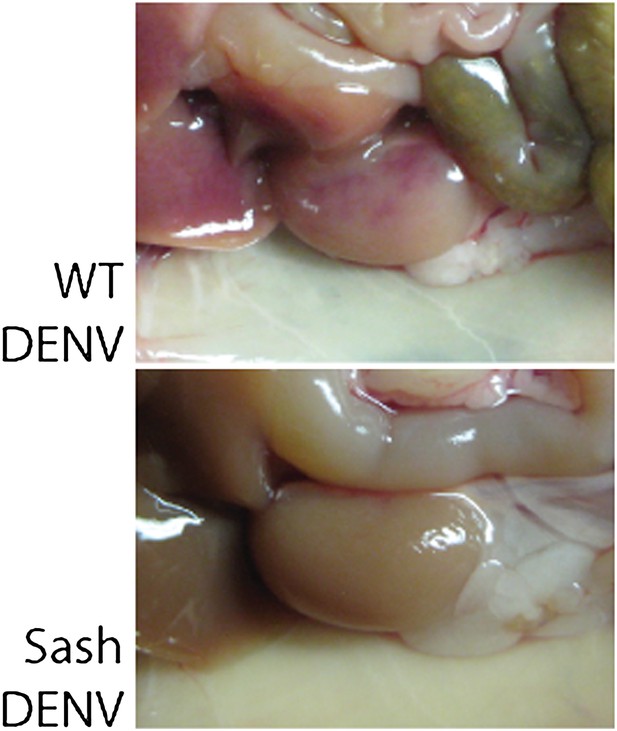
Representative images of the kidneys of WT mice or Sash mice that were infected with 1 × 106 PFU of DENV.
After injection of Evan's blue dye followed by saline perfusion, vascular leakage was visible in the kidneys of WT but not Sash mice after DENV infection.
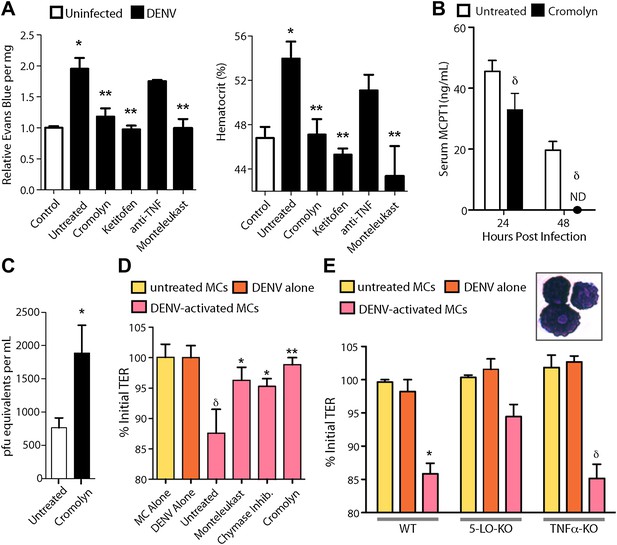
Drugs targeting MCs and their products improve DENV-induced vasculopathy.
(A) Evans blue dye perfusion studies and hematocrit analysis were performed to determine the vascular leakage in mice infected intra-peritoneally with 1 × 106 PFU of DENV. Serum was obtained from uninfected mice, DENV-infected and untreated mice, and mice that received MC-stabilizing or MC-product targeting treatments (see ‘Materials and methods’) 24 hr after infection. Error bars represent the SEM of values obtained from individual animals n = 3–6 per group. Data was analyzed by ANOVA with Bonferroni post-tests to determine significance; * indicates a significant increase over control (uninfected) values and ** indicates a significant decrease from DENV-infected, untreated values; p≤0.05. (B) Serum ELISA for MCPT1 was performed using pooled serum from DENV-infected, untreated mice and DENV-infected, cromolyn-treated mice. Significance was determined by ANOVA; δ indicates a significant decrease compared to untreated controls; p≤0.05. (C) Viral genome copies were quantified in the serum of mice infected with DENV that were either untreated or treated with cromolyn. The moderate increase with cromolyn treatment was not significant with p=0.09 and n = 5. (D) Trans-well assays demonstrate the direct activity of MCs and MC products on permeability of a monolayer of EOMA cells. Significance was determined by ANOVA. δ indicates a significant decrease in TER compared to exposure to supernatants from untreated MC or DENV alone treatment (p<0.05). Groups treated with montelukast or chymase inhibitor cocktail significantly increased TER over untreated EOMA cells exposed to supernatant from DENV activated MCs; *p<0.05. Cromolyn treatment during DENV exposure resulted in increased TER over supernatants from untreated DENV-exposed BMMCs **p<0.01. (E) Trans well assays were also performed using peritoneal and pleural cavity MCs isolated by antibody labeling and magnetic separation. Purified MCs, which have abundant eosinophilic cytoplasmic granules, are imaged in the inset. Purified MCs from WT, 5-LO-KO, or TNF-KO mice were untreated or treated with DENV (MOI = 5) for 1 hr prior to isolation of supernatant for exposure to EOMA cells. Supernatants from both WT and TNF-KO MCs resulted in a significant reduction in the TER of EOMA cells with exposure compared to controls, determined by ANOVA; for δ p<0.05. 5-LO-KO showed a trend towards slightly reduced TER, but this was not significant since p=0.06. DENV activated WT MCs promoted significantly reduced relative TER readings compared to DENV activated 5-LO-KO MCs, determined by t-test *p=0.01. Similar results were obtained in a second independent vascular endothelial cell line, SVEC4-10EHR1 (Figure 4—figure supplement 1).
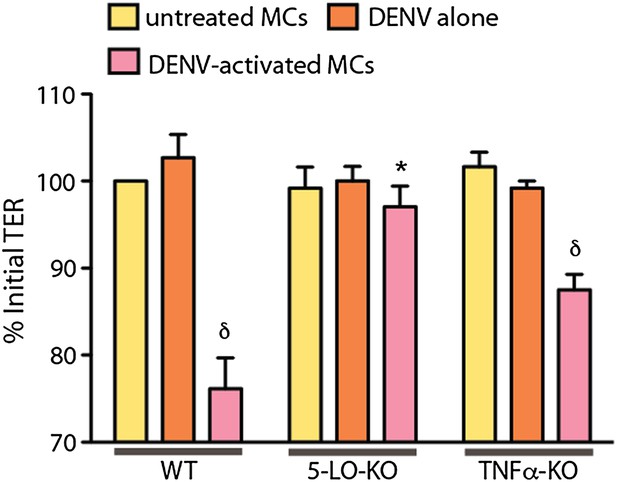
Supernatants from both WT and TNF-KO MCs resulted in a significant reduction in the TER of monolayers of vascular endothelial cell line SVEC4-10EHR1 with exposure compared to controls, determined by ANOVA; for δ p<0.05.
Supernatants from 5-LO-KO MCs did not promote detectable decreases in TER compared to controls. DENV activated WT MCs promoted significantly reduced relative TER readings compared to DENV activated 5-LO-KO MCs, determined by T-test *p=0.003, demonstrating that MC-derived leukotrienes mediate permeability of endothelial monolayers after DENV activation.
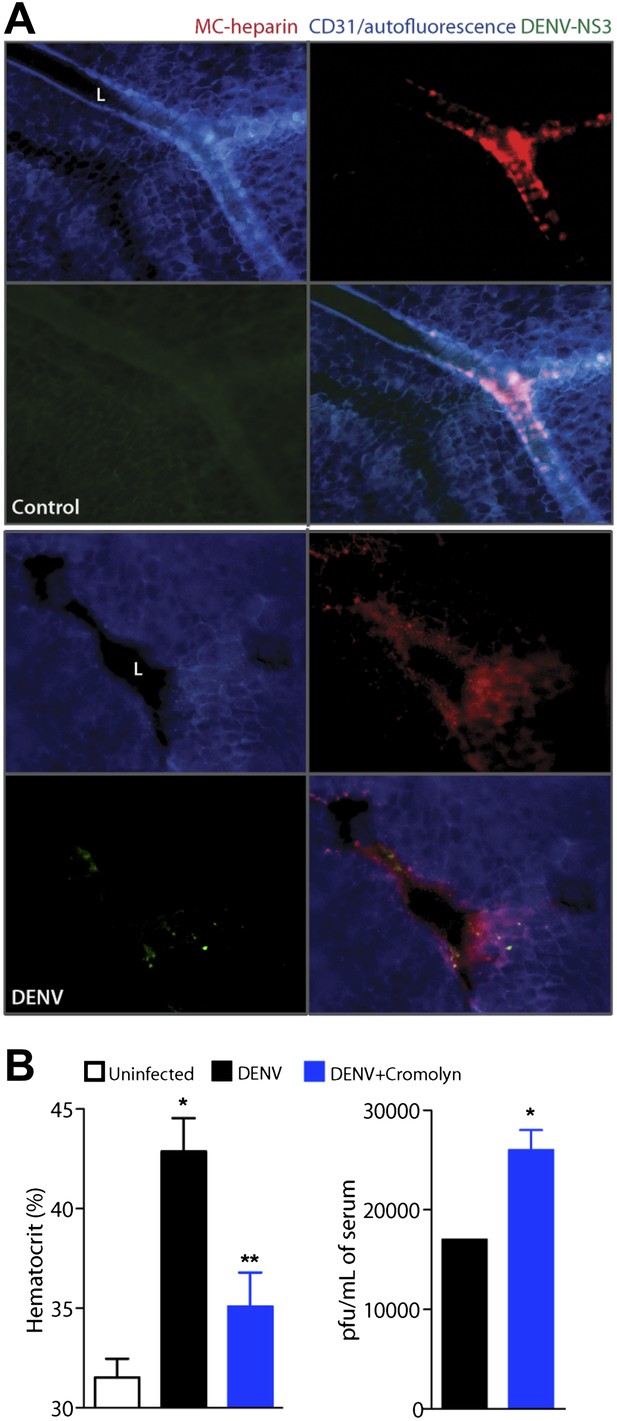
Cromolyn is effective in the IFN-α,β,γ-deficient mouse model to limit DENV-induced vasculopathy.
(A) Images are presented for control (top) and DENV-infected mesentery tissue (bottom) in channel series showing staining for blood vessels (CD31, blue), MC granules (MC-heparin by probing for Avidin, red), and viral replication (NS3, green), as well as the merged image. Mesentery tissue from the DENV-permissive mouse strain, AG129, was isolated from control or DENV-infected tissue at 24 hr after intra-peritoneally injection of 2 × 105 PFU of DENV strain Eden2, followed by immunostaining in whole mount and viewing at 20× magnification. MCs can be observed lining the blood vessels (branches of the mesenteric artery) in control tissue (left). Discrete avidin-staining particles suggest extensive degranulation in DENV-infected mesentery (right). Note that the endothelial junction marker, CD31, appears reduced and that NS3 staining is only present in the DENV-infected panel (right). L designates the lumen of the blood vessel in both panels. (B) Mice deficient in IFN-α,β,γ (strain AG129) were infected with DENV by intra-peritoneally injection of 2 × 105 PFU of Eden2. After 1 day, treatment was initiated for some infected mice by administering intra-peritoneally injections of cromolyn. On day 3, blood was collected from untreated and cromolyn-treated infected groups and uninfected controls. Hematocrit analysis was performed using blood from individual mice n ≥ 3. Error bars represent the SEM and * indicates a significant increase over uninfected controls and ** indicates a significant decrease compared to DENV infection alone. The p-value for the comparison between uninfected vs DENV + cromolyn was not significant. The graph in the right panel depicts the plaque forming units obtained using pooled serum. Error bars represent the SEM of the assay, which was performed in replicates. Where no error bars are apparent, values obtained were the same for each replicate. * indicates a significant increase for the cromolyn-treated animals compared to infection alone.
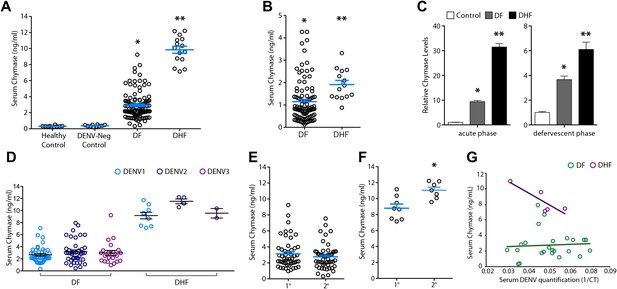
Severity of DENV-induced disease in humans is linked to the MC product chymase.
(A) Graph depicts the chymase concentration in human serum for healthy controls, DENV-negative febrile patients, and patients that were diagnosed with DF or DHF and positive for DENV by molecular tests (see ‘Materials and methods’). For DENV-Neg, DF and DHF patients, serum was collected during acute infection, 2–4 days after the onset of fever. (B) Graph depicts the serum chymase concentration in DF and DHF patients 4–7 days after fever onset (defervescent phase). For (A and B), each dot represents the average concentration for an individual patient (n = 10–108 patients per group). (C) Data is represented as the relative amount of chymase in patient samples obtained in the acute phase (left) or defervescent phase (right), after normalizing to the average chymase concentration in healthy control human serum. For (A–C) ANOVA analysis was used to determine significance of samples with Bonferroni’s post-test to determine significance between groups. * indicates a significant increase over healthy controls and DENV-Neg, febrile controls and ** indicates a significant increase over healthy control, DENV-Neg control, and DF groups. p<0.0001. (D) Graph depicts the concentration of chymase in serum samples grouped based on the serotype of DENV with which the patient was infected. Analysis by two-way ANOVA to compare chymase concentrations amongst DF and DHF samples reveals that serotype significantly influenced the chymase levels in patient sera, p<0.0001, although contributing to only 2.6% of the total variation. The concentrations of chymase in (E) DF or (F) DHF patients with either primary (1°) or secondary (2°) infection are shown. Chymase levels were significantly higher during secondary infection *p=0.0049 for DHF patients, but did not differ for DF patients, determined by Student’s unpaired t-test. (G) The concentrations of chymase are plotted vs the corresponding amounts of virus genome copies amplified from serum samples (represented as the inverse of the cross-over threshold [CT] value determined by real time PCR). For DF samples (green), Pearson’s R = 0.06, indicating no correlation. For DHF samples (purple), Pearson’s R = −0.85, indicating a correlation between higher viral genome copies and lower chymase levels.
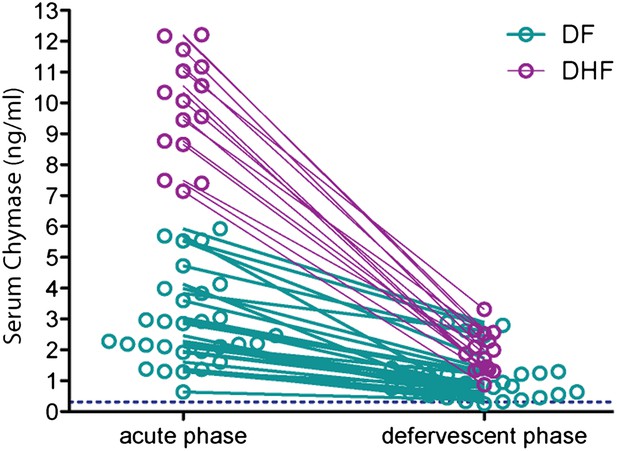
Serum chymase levels are presented to represent the repeated measures for individual patients at the early (acute) and late (defervescent) time points of infection and a line connects each patient’s paired values.
When assessed using a repeated measures ANOVA, DF and DHF groups differ highly significantly with p<0.0001. To aid visualization of paired data, the DF cohort data set was truncated at patient 30 and data symbols were ‘nudged’ using prism software to prevent overlap.
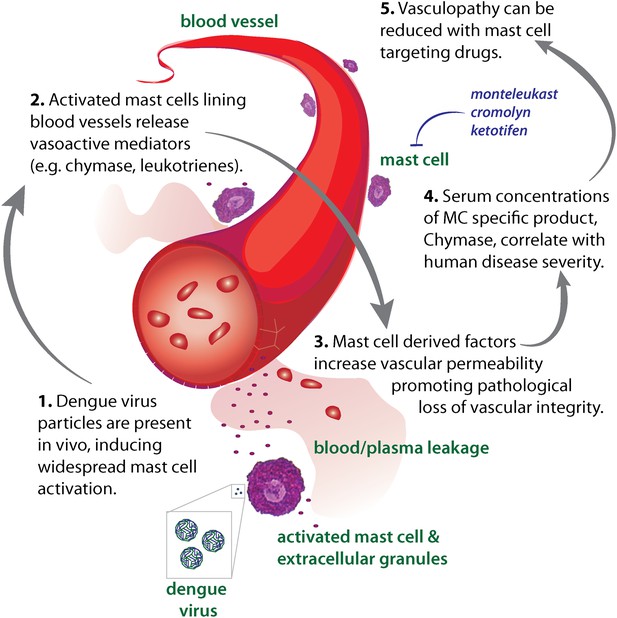
Diagram representing the impact of DENV-induced activation of MCs on the vasculature.
High DENV viral titers in vivo results in the activation of MCs, which release many vasoactive factors in the vicinity of blood vessels including leukotrienes and proteases, such as chymase. These factors act in concert to promote vascular leakage that, when occurring on a systemic level, has pathological consequences for the host. Drugs that target MC products can limit this leakage and vascular pathology. Similarly, the MC-specific product chymase can also be used to predict the severity of hemorrhagic disease in human patients.





