Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2
Figures
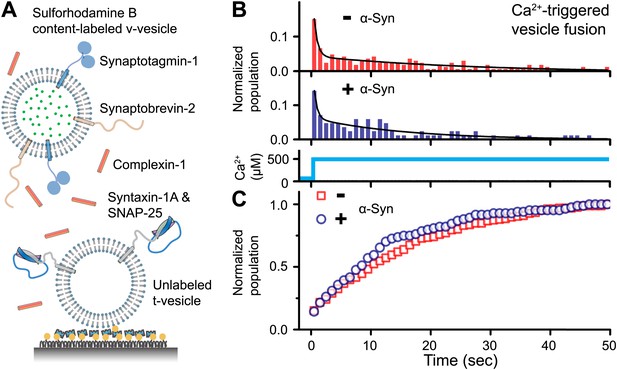
Native α-Syn has no effect on Ca2+ -triggered v-/t-vesicle fusion.
(A) Experimental scheme of our single v-/t-vesicle content mixing system (Kyoung et al., 2011; Kyoung et al., 2012), with improvements described in (Diao et al., 2012a), and further modifications described in ‘Materials and methods’. (B) and (C) α-Syn has little effect on the probability of triggered vesicle fusion upon injection of 500 μM Ca2+. Panels show histograms of the occurrence (B) and the corresponding cumulative distribution (C) of Ca2+-triggered complete fusion (content mixing) in presence or absence of 2 μM α-Syn. In the absence of α-Syn, we observed 166 fusion events out of ∼2000 docked v-vesicles (identified as fluorescent spots that were present prior to Ca2+ injection), whereas in the presence of α-Syn, we observed 84 fusion events out of ∼1300 docked v-vesicles within the observation period of 50 s. The time-binning was 1 s. Black lines are fits to bi-exponential decay functions over the entire observation period of 50 s. In the absence of α-Syn, the fitted function is f(t) = −0.0046 + 0.048 e-t/27.1 + 0.26 e-t/0.58, whereas in the presence of α-Syn, the fitted function is f(t) = −0.001 + 0.064 e-t/15.4 + 0.22 e-t/0.51 with t in sec.
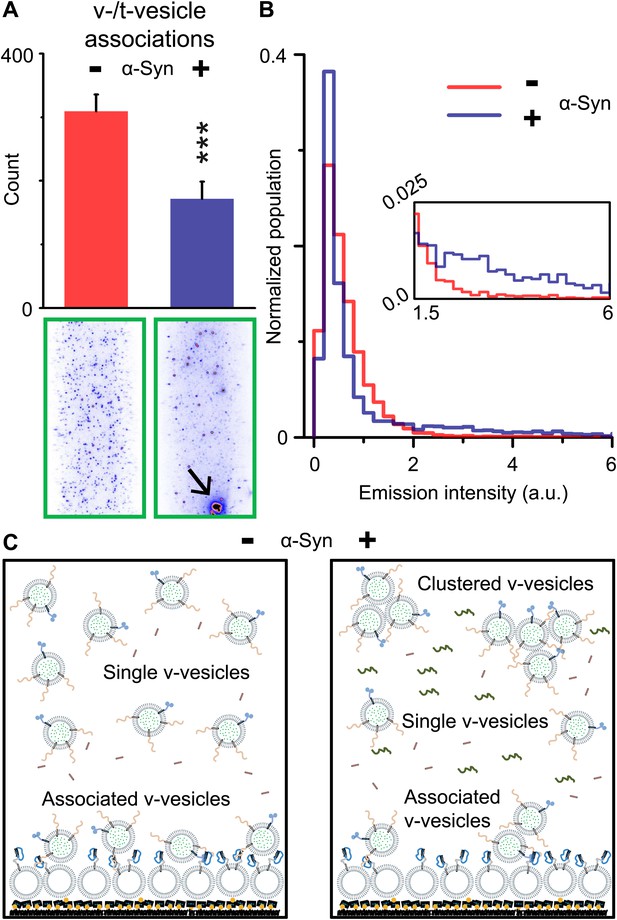
Native α-Syn decreases the number of v-/t-vesicle associations.
(A) Plotted is the number of content dye fluorescent spots in presence of 2 μM complexin-1 and with or without 2 μM α-Syn (tag-free construct); example images are shown in the lower two panels. Scoring (‘counts’) excluded the few very large fluorescent spots that appeared in the presence of α-Syn; an example is shown in the lower right panel, marked by a black arrow. Error bars are standard deviations from 10 random imaging locations within the same sample channel. *** indicates p<0.001 by the Student's t-test. (B) Distribution of the fluorescence intensity of all fluorescent spots as shown in panel (A). In the presence of α-Syn, the number of low intensity fluorescent spots (up to 1.5 a.u.) decreased, which was accompanied by an increase in higher intensity fluorescent spots (see inset) due to the formation of v-vesicle clusters. The very large fluorescent spot (black arrow in the lower right image in panel (A)) was excluded in this distribution. (C) Illustration that clustering of v-vesicles induced by α-Syn reduces the effective number of fluorescent spots (corresponding to docked single or multiples of v-vesicles) in a finite volume since the starting concentration of individual v-vesicles was identical with and without α-Syn.
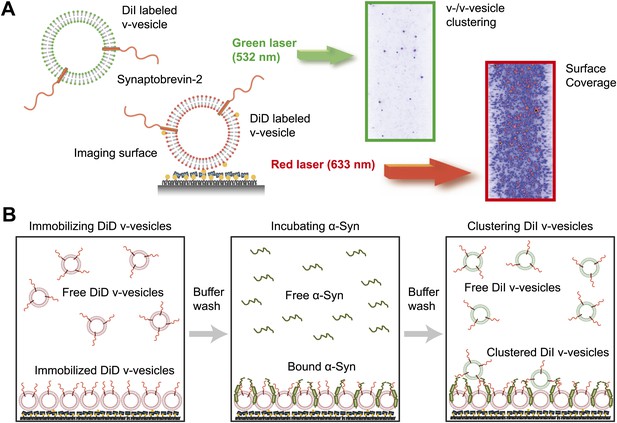
Native α-Syn induces clustering of synaptobrevin-2 v-vesicles.
(A) Experimental scheme of our single-vesicle assay for monitoring clustering of synaptobrevin-2 v-vesicles. A saturated layer of DiD-labeled v-vesicles with reconstituted synaptobrevin-2 was immobilized on an imaging surface via biotin/neutravidin interactions. Free-floating DiI-labeled vesicles with reconstituted synaptobrevin-2 were injected into the sample chamber (‘Materials and methods’). The number of v-/v-vesicle interactions (clustering) in presence or absence of α-Syn was determined by counting the number of spots arising from fluorescence emission of DiI upon excitation at 532 nm. (B) Experimental flow: 1. Immobilization of DiD-labeled v-vesicles on the imaging surface through biotin/neutravidin interactions. 2. Buffer exchange. 3. α-Syn incubation of the surface with immobilized DiD v-vesicles. 4. Buffer exchange, removing unbound or weakly bound α-Syn molecules. 5. Injection of DiI-labeled v-vesicles without α-Syn. Following another buffer exchange, the channels were imaged on the microscope.
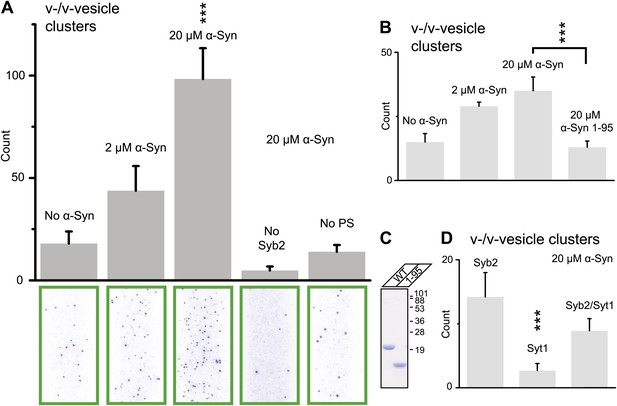
Native α-Syn (tag-free construct) promotes v-vesicle clustering by binding to both synaptobrevin-2 and anionic membranes.
(A) α-Syn increases the number of interacting DiI-labeled v-vesicles on the imaging surface in a concentration-dependent manner. Bar graph: quantitation of interacting vesicles. Bottom panel: representative fluorescence images of interacting vesicles on the imaging surface. Error bars are standard deviations from 15 random imaging locations in the sample channel obtained. (B) Number of interactions between free-floating and immobilized v-vesicles in presence of wildtype α-Syn and its mutant that does not bind to synaptobrevin-2 (α-Syn[1-95] with myc-tag) at indicated concentrations. (C) Purified α-Syn wildtype and α-Syn[1-95] (with myc-tag) expressed in bacteria were analyzed by SDS-PAGE and Coomassie staining. (D) Number of interactions in the presence of 20 μM α-Syn (myc-tag construct). At variance to panels (A, B, and C), both free-floating and immobilized v-vesicles were reconstituted with synaptobrevin-2 (Syb2), synaptotagmin-1 (Syt1), or both (Syb2/Syt1). Note, that the slight variation in the ‘absolute’ number of observed fluorescent spots between comparable experiments in panels (A, B, and D) arises mainly from tagged vs untagged versions of α-Syn, as well as from different liposome and imaging surface preparations. Yet, the ‘relative’ differences were statistically similar for different protein preparations. In all panels, error bars are standard deviations from 10 random imaging locations in the same sample channel, and *** indicates p<0.001 by the Student's t-test.
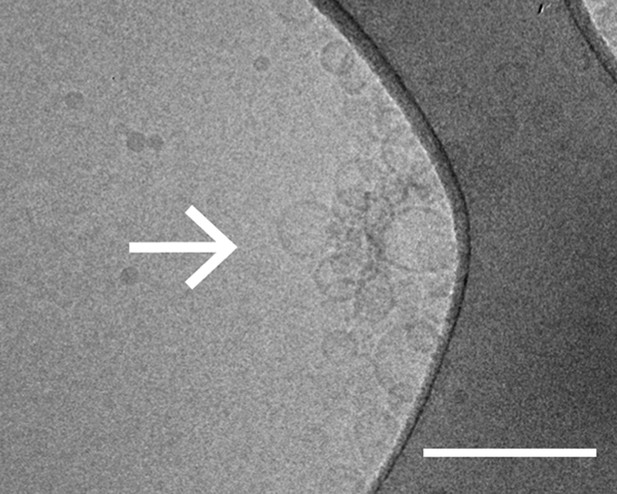
Cryo-electron microscopy image of clusters of synaptobrevin-2 v-vesicles as induced by native α-Syn. Scale bar is 200 nm.
The dark feature is the holey carbon grid. In contrast, reconstituted v-vesicles in the absence of α-Syn do not form clusters (Kyoung et al., 2011).
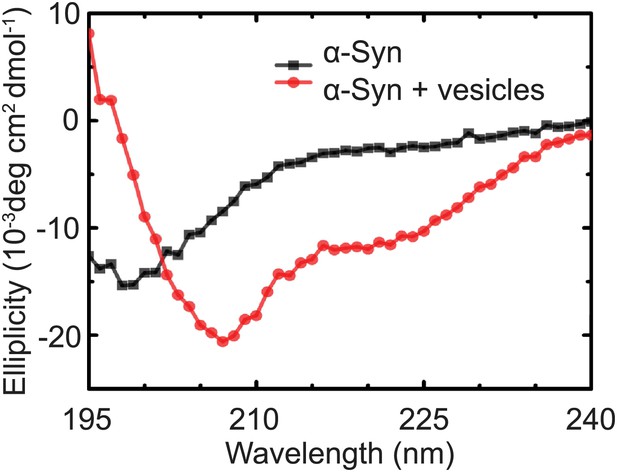
Native α-Syn undergoes a conformational transition from a predominantly unstructured state in solution to an α-helical state upon binding to protein-free vesicles as measured by CD spectroscopy.
The protein-free vesicles had the same lipid composition as the v-vesicles used throughout this work. The molar protein-to-lipid ratio was 1:530, and tag-free wildtype α-Syn was used.
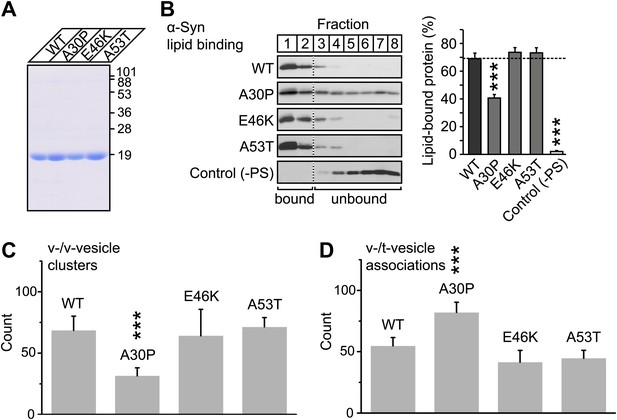
V-vesicle clustering correlates with lipid binding of native α-Syn.
(A) Purification of α-Syn expressed in bacteria. Purified α-Syn wildtype and the three Parkinson's disease (PD)-related mutants A30P, E46K, and A53T (all tag-free constructs) were analyzed by SDS-PAGE and Coomassie staining. (B) Lipid binding of recombinant α-Syn wildtype, and of the PD mutants A30P, E46K, and A53T. α-Syn was incubated with negatively charged liposomes (30% phosphatidylserine, 70% phosphatidylcholine, or 100% phosphatidylcholine as control), and subjected to a flotation assay. Eight fractions were collected from top to bottom of the flotation gradient, and equal volumes of each fraction were separated by SDS-PAGE and immunoblotted for α-Syn. Top two fractions were defined as lipid-bound, and quantitated as percent of total α-Syn. Data shown are means ± SEM; n = 3. (C) Impaired lipid-binding of α-Syn decreases v-vesicle clustering. In order to measure vesicle clustering, the number of DiI-labeled v-vesicles that were docked to immobilized v-vesicles was counted, as illustrated in Figure 3. (D) Impaired lipid-binding of α-Syn increases the number of v-/t-vesicle associations. The number of fluorescent spots arising from labeled v-vesicles were counted that were docked to t-vesicles (immobilized to the imaging-surface), as illustrated in Figure 2, but in the presence of the specified α-Syn mutants without complexin-1. In all panels, error bars are standard deviations from 10 random imaging locations in the same sample channel, and *** indicates p<0.001 by the Student's t-test.
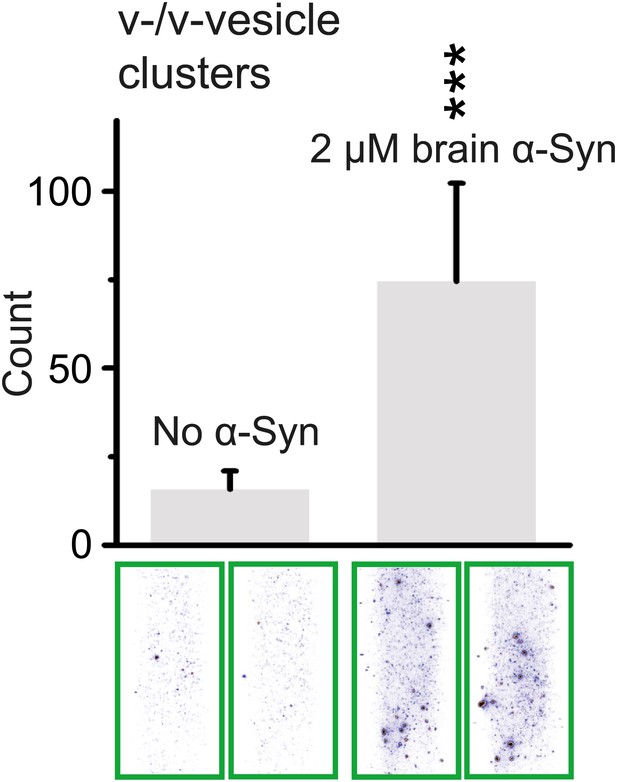
Native α-Syn purified from mouse brain induces v-vesicle clustering.
Error bars are standard deviations from 20 random imaging locations in the same sample channel. *** indicates p<0.001 by the Student's t-test.
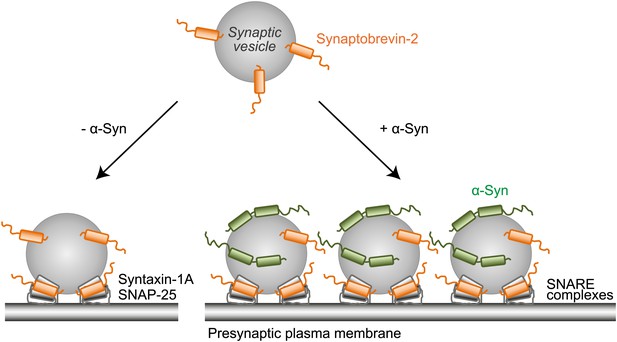
Proposed model of native α-Syn function.
Native α-Syn binds at the same time to the synaptic vesicle membrane and synaptobrevin-2. Synaptic vesicle fusion with the presynaptic plasma membrane is mediated by the three neuronal SNARE proteins synaptobrevin-2 (on the synaptic vesicle membrane), SNAP-25 and syntaxin-1 (on the presynaptic plasma membrane), which form the synaptic SNARE-complex. Native α-Syn clusters synaptic vesicles depending on binding to synaptobrevin-2 and the vesicles themselves, which may result in a local increase of synaptic vesicles at the presynaptic plasma membrane, and a subsequent increase in SNARE-complex formation.





