Slo1 is the principal potassium channel of human spermatozoa
Figures
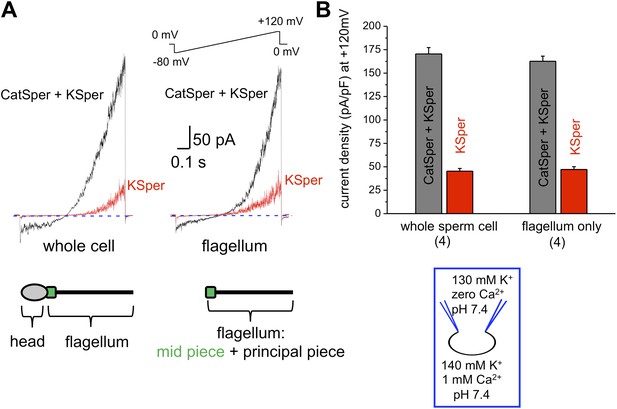
hKSper currents originate from the sperm tail.
(A) IKSper was recorded in response to a voltage ramp as shown. Shown are representative traces from whole spermatozoon (left panel; recordings are from the same cell) and sperm tail (right panel; recordings are from the same flagellum). Black traces represent currents in divalent free conditions, which allow K+ current through CatSper. Red traces show true IKSper. Latter was recorded in the presence of 1 mM extracellular Ca2+, which inhibits monovalent currents through CatSper. (B) Current densities were obtained at +120 mV and presented as mean ± SEM. (n), number of experiments. Four different sperm cells (or four different sperm flagella) of two different human donors were used.
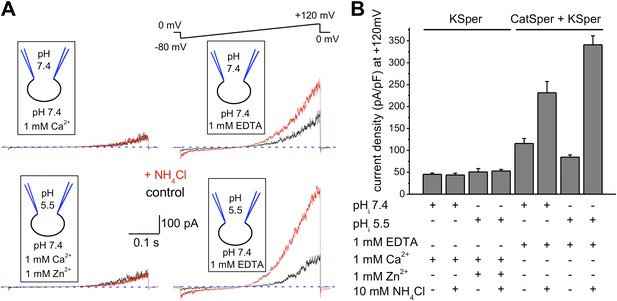
hKSper currents are insensitive to intracellular alkalinization.
(A) Representative KSper currents were recorded from sperm cells in response to voltage ramps as shown. Recordings were done with various pHi as indicated. The bath solution containing 1 mM Ca2+ was used to inhibit K+ current through CatSper (left panels). Right panels show traces in divalent free conditions, which allow K+ current through CatSper. Intracellular alkalinization was evoked by addition of 10 mM NH4Cl to the bath (red traces). A weak intracellular buffer (5 mM of HEPES or MES) allowed instantaneous pH changes. Zn2+ was used to block H+ currents via Hv1 at acidic intracellular pH. The upper panels and the lower panels are recordings from two different sperm cells. (B) KSper and CatSper/KSper current densities (CDs) recorded from sperm cells as shown in (A). At pHi 7.4 KSper CDs were: 45 ± 3 pA/pF (control) and 44 ± 4 pA/pF (plus NH4Cl). These values were similar at pHi 5.5: CDs were: 51 ± 8 pA/pF (control) and 53 ± 4 pA/pF (plus NH4Cl). However, under DVF conditions that permit K+ efflux through CatSper, CDs at pHi 7.4 were: 116 ± 11 pA/pF (control) and 231 ± 26 pA/pF (plus NH4Cl). At pHi 5.5, CDs were: 85 ± 5 pA/pF (control) and 341 ± 21 pA/pF (plus NH4Cl). Shown are CDs acquired at +120 mV and presented as mean ± SEM; n = 4–6 independent experiments with cells from four different human donors.
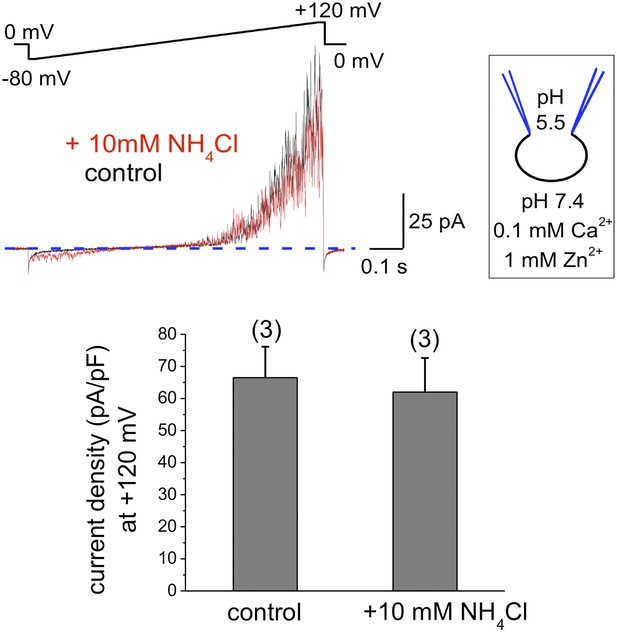
hKSper currents from human epididymal spermatozoa are insensitive to intracellular alkalinization.
The upper panel shows representative IKSper traces recorded from human epididymal spermatozoa (whole sperm cell) in the control (black) and in the presence of 10 mM NH4Cl (red). The lower panel presents mean currents acquired at +120 mV; (n), number of experiments. IKSper did not change upon intracellular alkalinization with current densities averaging at 67 ± 10 pA (control) and 62 ± 11 pA (after addition of 10 mM NH4Cl). Three epididymal spermatozoa were tested.
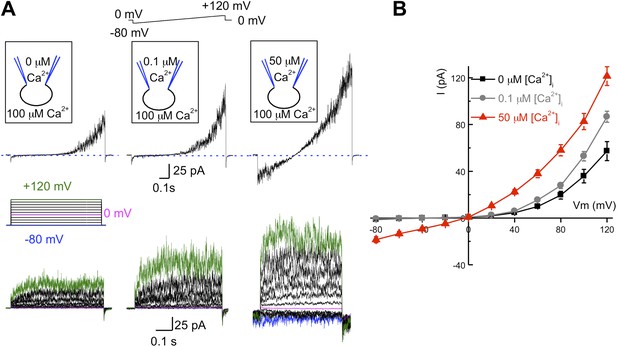
hKSper is activated by intracellular calcium.
(A) Upper panels: representative IKSper recorded with various intracellular [Ca2+]free as indicated, in response to a voltage ramp. Lower panels: corresponding representative IKSper elicited by a step protocol from a holding potential of −80 mV to +120 mV with 20 mV increments. For clarity, traces at −80 mV, 0 mV, and +120 mV are labeled in blue, magenta, and green, respectively. Representative traces were obtained from three different sperm cells (upper and lower panels). (B) Current–voltage (I–V) relationship in response to 0 μM (black), 0.1 μM (gray), and 50 μM (red) intracellular [Ca2+]free. At a membrane potential (Vm) of −80 mV, potassium currents were: -1.2 ± 0.5 pA ([Ca2+]i = 0), -0.8 ± 0.2 pA ([Ca2+]i = 0.1 μM), and -18.5 ± 2.6 pA ([Ca2+]i = 50 μM). At Vm = +120 mV, IKSpers were 57 ± 8 pA ([Ca2+]i = 0), 87 ± 5 pA ([Ca2+]i = 0.1 μM), and 122 ± 8 pA ([Ca2+]i = 50 μM). Data are shown as means ± SEM; n = 6–11 independent experiments with cells from six different donors. Data are from whole sperm cells.
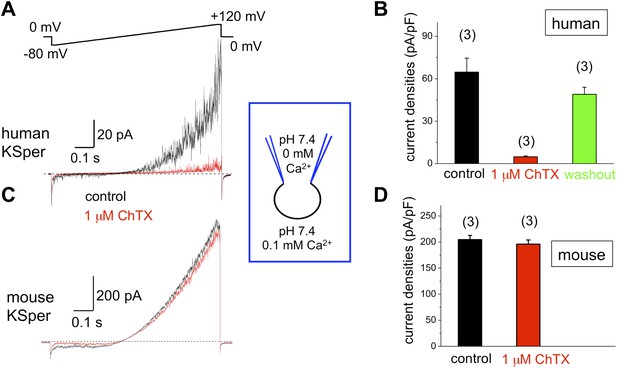
Human, but not mouse KSper is sensitive to the Slo1 channel blocker charybdotoxin (ChTX).
(A) Representative human IKSper traces under control conditions (black) and in the presence of 1 μM ChTX (red) elicited in response to the given voltage ramp. (B) Mean current densities (CDs) ± SEM calculated at +120 mV. CDs (human) were: 65 ± 10 pA/pF (control), 5 ± 1 pA/pF (ChTX), and 49 ± 5 pA/pF (washout). (C) Representative mouse IKSper traces under control conditions (black) and in the presence of 1 μM ChTX (red) elicited in response to the voltage ramp as shown in (A). (D) CDs (mouse) were: 205 ± 8 pA/pF (control) vs 196 ± 8 pA/pF (ChTX). (n), number of experiments. Three human and three mouse sperm cells were used.
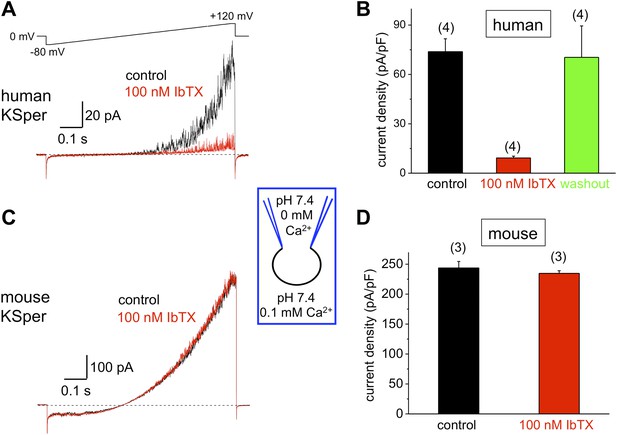
Human, but not mouse KSper is sensitive to the Slo1 channel blocker iberiotoxin (IbTX).
(A) Representative human IKSper traces under control conditions (black) and in the presence of 100 nM IbTX (red) elicited in response to the shown voltage ramp. (B) Mean current densities (CDs) ± SEM calculated at +120 mV. CDs (human) were 74 ± 8 pA/pF (control), 9 ± 1 pA/pF (IbTX), and 70 ± 19 pA/pF (washout). (C) Representative mouse IKSper traces under control conditions (black) and in the presence of 100 nM IbTX (red) elicited in response to the voltage ramp as in (A). (D) CDs (mouse) were 244 ± 11 pA/pF (control) and 235 ± 4 pA/pF (IbTX). (n), number of experiments. Four human and three mouse sperm cells were used.
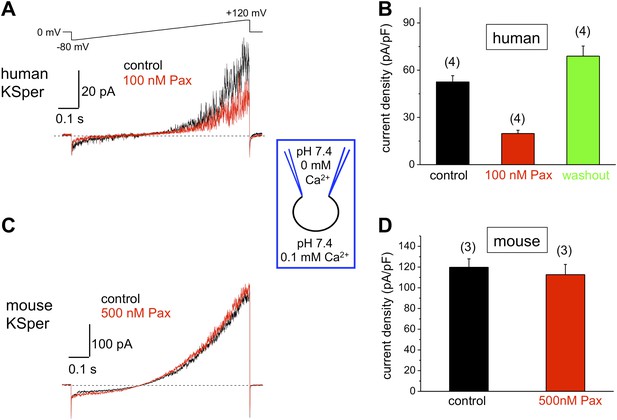
Human, but not mouse KSper is sensitive to the Slo1 channel blocker paxilline (Pax).
(A) Representative human IKSper traces under control conditions and in the presence of paxilline elicited in response to the indicated voltage ramp. (B) Mean current densities (CDs) ± SEM calculated at +120 mV. Cells from three donors were used. CDs (human) were: 53 ± 4 pA (control), 20 ± 2 pA (100 nM paxilline), and 69 ± 6 pA (washout). (C) Representative mouse IKSper traces under control conditions and in the presence of paxilline elicited in response to the voltage ramp as in (A). (D) CDs (mouse) were: 119 ± 5 pA/pF (control) and 113 ± 10 pA/pF (500 nM paxilline). (n), number of experiments. Four human and three mouse sperm cells were used.
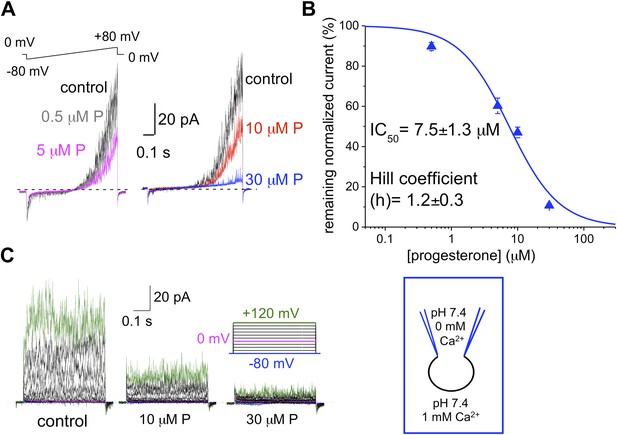
hKSper is blocked dose-dependently by progesterone (P).
(A) Representative IKSper recordings from two sperm cells (left and right panel) in response to the given voltage ramp protocol under control conditions (black), 0.5 μM P (gray), 5 μM P (magenta), 10 μM P (red), and 30 μM P (blue). (B) Dose-dependent inhibition of human IKSper by progesterone. Human IKSper amplitudes were acquired at +80 mV at the end of the voltage ramps, as shown in (A). Current amplitudes in the presence of indicated progesterone concentrations were normalized onto control amplitudes (in the absence of progesterone). Remaining IKSper in the presence of 0.5 μM, 5 μM, 10 μM and 30 μM of P was: 90 ± 2%, 60 ± 4%, 47 ± 3% and 11 ± 1%, respectively. Data were fitted with the Hill equation. Data shown are means ± SEM of 4–10 sperm cells from three different donors. (C) Representative IKSper traces elicited by the given voltage step protocol of the control (left panel) and in the presence of 10 μM P (middle panel) and 30 μM P (right panel). Recordings are from the same cell as in (A).
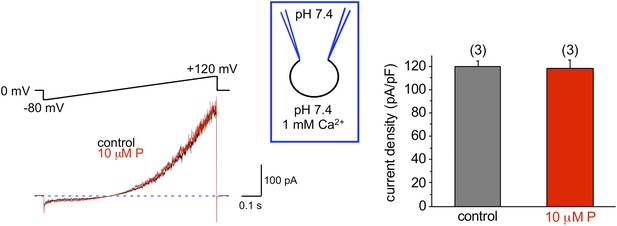
Mouse KSper is insensitive to progesterone (P).
The left panel shows representative traces of mouse IKSper of the control (black) and in the presence of 10 μM P (red). The right panel shows current densities (CDs) acquired at +120 mV presented as mean ± SEM. CDs were: 119 ± 5 pA/pF (control) and 118 ± 8 pA/pF (10 μM P). (n), number of experiments. Three sperm cells were used.
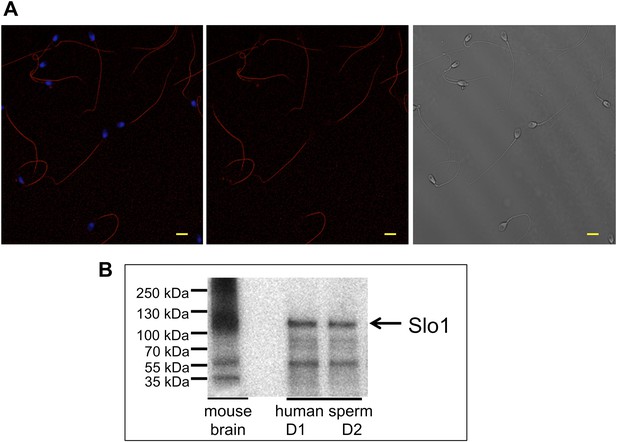
Slo1 protein is present in human spermatozoa.
(A) Human sperm immunostaining with primary polyclonal anti-Slo1 antibodies and Cy3-conjugated secondary antibodies. Left and middle panels show Slo1 staining localized to the principal piece of human sperm flagellum. Left panel: nuclei are stained by DAPI. Right panel: DIC image of the same cells. Scale bar is 5 mm. (B) Representative immunoblot of the mouse brain (positive control) and human spermatozoa from two different donors (donor 1 and donor 2: D1 and D2, respectively).
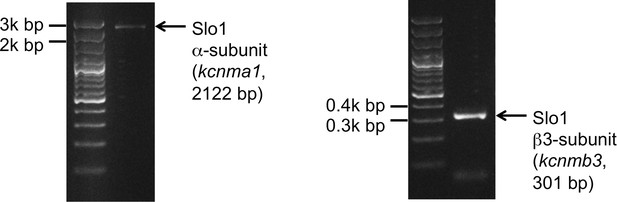
Slo1 transcripts are present in human spermatozoa.
PCR bands of the portion of the translated region of kcnma1 (left panel; 1433–3554 bp, corresponding to the coding sequence of splice isoform1; UniProt # Q12791), and of the translated region of kcnmb3 (right panel; 529–829 bp of the coding sequence of splice isoform 3d, Uniprot # Q9NPA1).
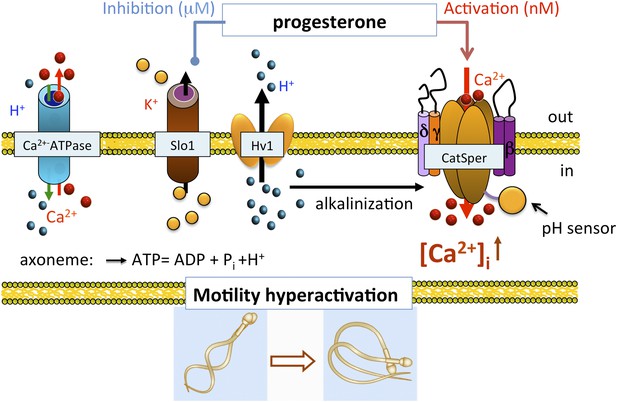
Role of human KSper (Slo1) in sperm physiology.
In the uterus and fallopian tube, CatSper is partially activated due to the intracellular alkalinization evoked by proton extrusion through Hv1 and picomolar- to nanomolar progesterone (P) concentrations. However, to achieve full activation of CatSper, flagellar plasma membrane must be depolarized. This is achieved by the inhibition of sperm KSper, the channel responsible for membrane hyperpolarization. In close proximity to the oocyte, spermatozoa encounter micromolar concentrations of P, which inhibit hKSper, resulting in membrane depolarization. These events allow full activation of CatSper, trigger sperm hyperactivation, allow spermatozoa to penetrate through the egg protective vestment, and make fertilization possible.
Videos
Inhibition of hKSper induces a hyperactivation- like motility pattern.
Normal motility of human spermatozoa in the control HS solution. Scale bar is 5 mm. Recording was slowed down five times.
Inhibition of hKSper induces a hyperactivation- like motility pattern.
Motility of human spermatozoa is altered after incubation in HS solution, which contained 100 nM of charybdotoxin (ChTX). Scale bar is 5 mm. Recording was slowed down five times.





