Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts
Figures
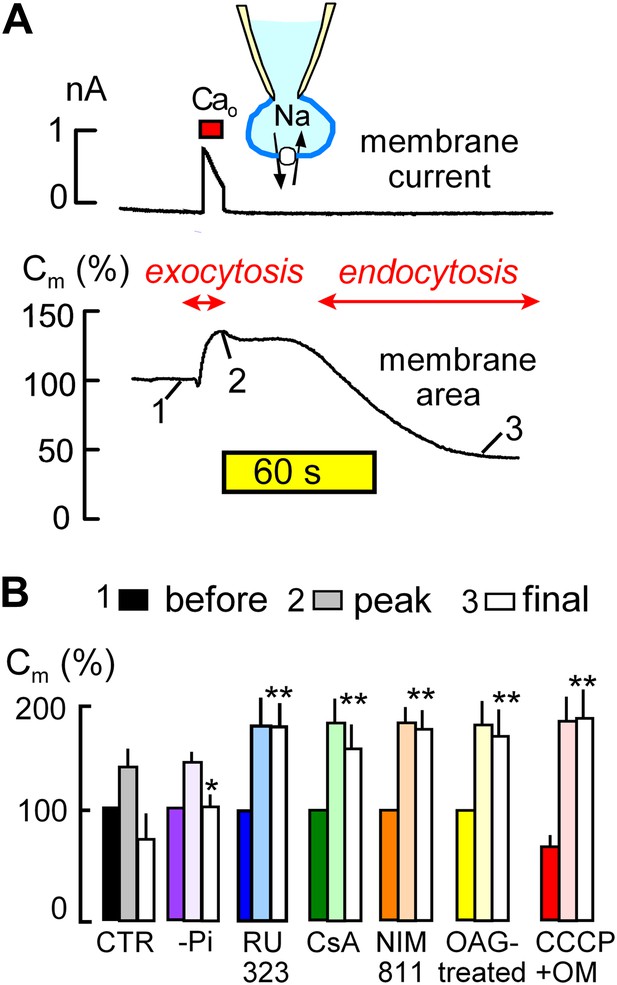
MEND is initiated by mitochondria.
(A) Records of membrane current (above) and electrical membrane capacitance (below) during the Standard MEND protocol in BHK fibroblasts expressing cardiac Na/Ca exchangers (NCX1). Cells are opened in Ca-free extracellular solution with 0.5 mM EGTA, using a cytoplasmic (pipette) solution containing 40 mM cytoplasmic Na, 8 mM MgATP and 0.2 mM GTP. Thereafter, Ca influx via Na/Ca exchange is activated by extracellular application of 3 mM Ca for 10 s. During Ca influx, outward membrane current reflects 3Na/1 Ca exchange. Membrane area (i.e., Cm) increases by 30% as a result of exocytosis (i.e., fusion of vesicles to the cell surface). After terminating Ca influx, membrane area is stable for nearly 60 s and then declines over 2 min by 70% as plasmalemma is internalized via endocytosis. (B) Composite results implicating a role for mitochondria in the initiation of MEND. From left to right, bar graphs give results for Standard MEND (CTR, black and white), Standard MEND without cytoplasmic Pi (purple), with the mitochondrial Ca uptake inhibitor, RU323 (20 μM, blue), with the PTP blockers, cyclosporine A (5 μM, green) and NIM811 (2 μM, orange), after PKC activation by OAG (15 μM, yellow), and after rapid perfusion of a mitochondrial uncoupler, CCCP (20 μM) with an ATP synthetase inhibitor, oligomycin (OM, 5 μM, red). n > 6 in all panels. MEND was quantified as fractional decrease of Cm from point 2 to point 3, and stars indicating significance have their usual meanings.
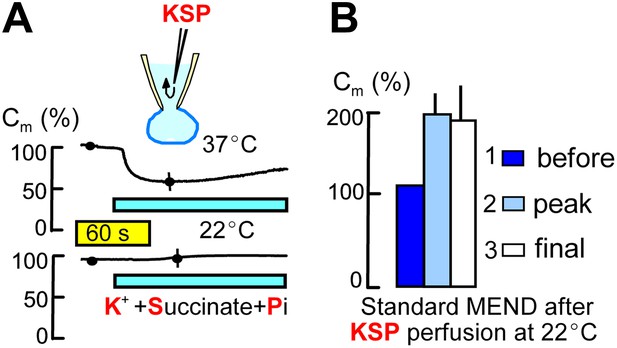
Metabolites that promote PTP openings rapidly initiate MEND.
Experiments were started with Pi-free cytoplasmic solution. Subsequently, rapid internal perfusion of cells was initiated via a silica mico-capillary tube whose orifice was placed within 50 μm of the patch pipette orifice. KSP solution was prepared by replacing 80 mM NMG and 20 mM TEA in the standard internal solution with 100 mM K. In addition, 5 mM succinate and 1 mM Pi were added, and free Ca was buffered to 0.25 μM with 2 mM EGTA. (A) The upper record illustrates MEND responses recorded at 37°C upon cytoplasmic perfusion of KSP solution, and the lower record illustrates the failure of MEND to occur at 22°C. (B) Composite results for experiments in which the plasmalemma patch within the patch pipette was ruptured by suction (i.e., the cell was ‘opened’) at 22°C with KSP solution in the pipette, and the Standard MEND protocol was performed 1 to 2 min later at 37°C. n > 6 for all results.
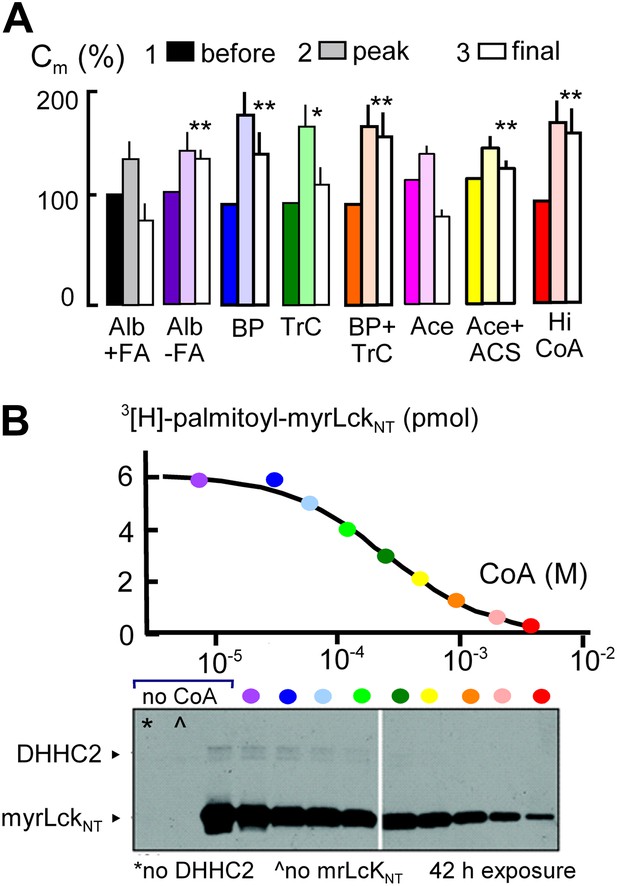
MEND is blocked by preventing protein palmitoylation reactions.
(A) From left to right, composite results for Standard MEND after incubating cells with 1:1 palmitate-loaded albumin (50 μM) for 1 hr (black), after incubating cells with fatty acid-free albumin (Alb, 50 μM) for 1 hr (purple), with the palmitoylation inhibitor, bromopalmitate (BP, 50 μM) included in all solutions (blue), with the acyl CoA synthetase inhibitor, Triascin C (TrC, 2 μM) in all solutions (green), with BP (50 μM) and TrC (2 μM) in all solutions (orange), with cytoplasmic acetate (Ace, 6 mM; pink), with acetate (6 mM) and acetyl CoA synthetase (ACS, 20 μM; yellow), and with a high cytoplasmic CoA concentration (3 mM) to block DHHCs (red). For all results, n > 6. (B) CoA inhibition of DHHC2-mediated palmitoylation of the N-terminus of myristoylated lymphocyte-specific kinase (myrLckNT). For details, see ‘Materials and methods’.
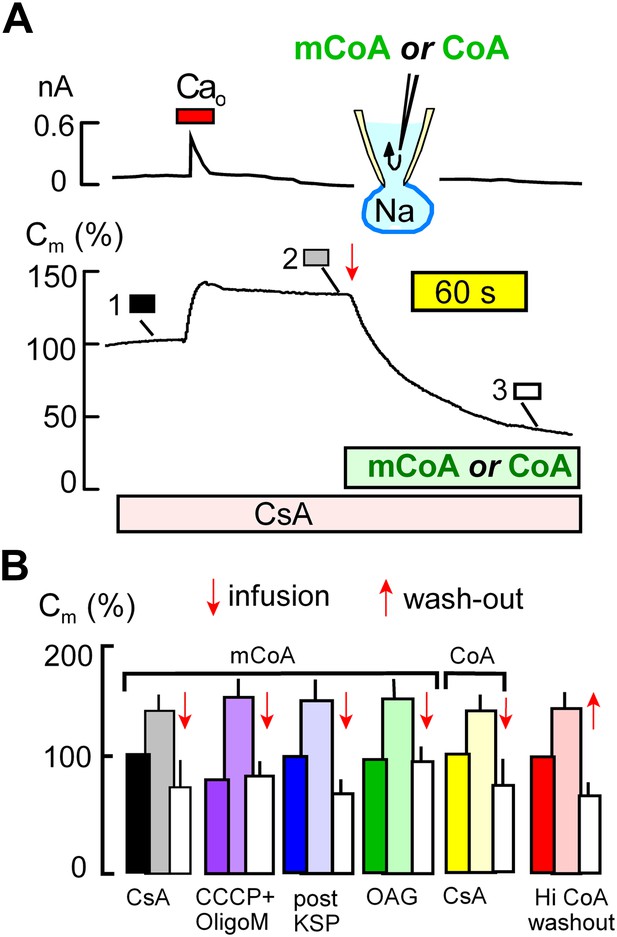
Cytoplasmic perfusion of acyl CoA or CoA circumvents four mitochondrial MEND blocks.
(A) Typical experiment in the presence of cyclosporine A (CsA, 3 μM) to block MEND. Ca influx cause a 40% increase of Cm via exocytosis, and Cm remains stable thereafter for minutes. Cytoplasmic perfusion of myristoyl CoA (mCoA, 15 μM) via the micro-capillary within the patch pipette causes a MEND response that begins within 10 s and internalizes 70% of the plasmalemma within 2.5 min. (B) Composite results quantifying MEND that occurs when mCoA or CoA is perfused into the cytoplasm of cells in which MEND has been blocked by interventions acting on mitochondria. From left to right, bar graphs present results for pipette perfusion of mCoA (15 μM) in cells in which MEND was blocked by cyclosporine A (CsA, 3 μM; black and white), by opening cells at 22°C with CCCP (20 μM) and oligomycin (5 μM; purple), by opening cells at 22°C with KSP (blue), and by pretreatment of cells with OAG (15 μM) for 30 min (green). The penultimate results quantify MEND caused by pipette perfusion of CoA (20 μM) into cyclosporine A-blocked cells (yellow). The final data set shows results for cells in which MEND was blocked by a high cytoplasmic CoA concentration (3 mM). Ca influx caused on average 38% exocytic responses, and Cm was then stable. When CoA was perfused out to the cytoplasm (‘wash-out’), endocytosis started within 15 s and amounted to 56% of the plasmalemma on average. For all results, n > 6.
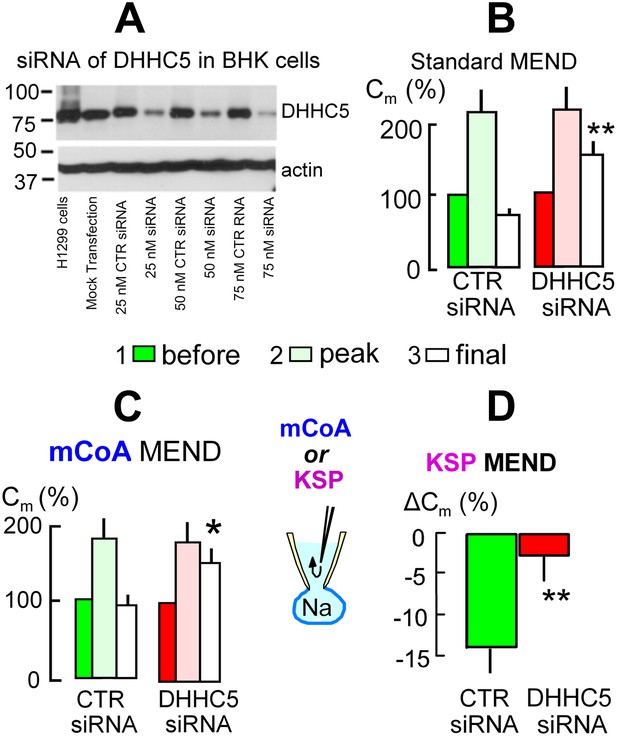
The acyl tranferase, DHHC5, is required for MEND in BHK cells.
(A) DHHC5 knockdown by siRNA to ZDHHC5. BHK cells were transfected with control siRNA or ZDHHC5-specific siRNA at indicated concentrations using Lipofectamine 2000. 72 hrs after transfection, cells were harvested and cell lysates were processed for Western blot analysis using 20 µg protein per lane. A human non-small lung carcinoma cell line, H1299, was used as a positive control for anti-ZDHHC5 antibody. (B) MEND in BHK cells amounts to 75% of the cell surface after growing cells with staurosporin (0.1 μM), and DHHC5 siRNA decreases MEND by 63% (p<0.01). (C) mCoA-relief of MEND block by cyclosporine A (3 μM) is reduced by 75% (p<0.05) in BHK cells transfected with siRNA for DHHC5 vs scrambled siRNA. (D) KSP-induced MEND in BHK cells is reduced from 14 to 2.5% by DHHC5 siRNA transfection using RNAmax (p<0.01). For all results, n > 6.
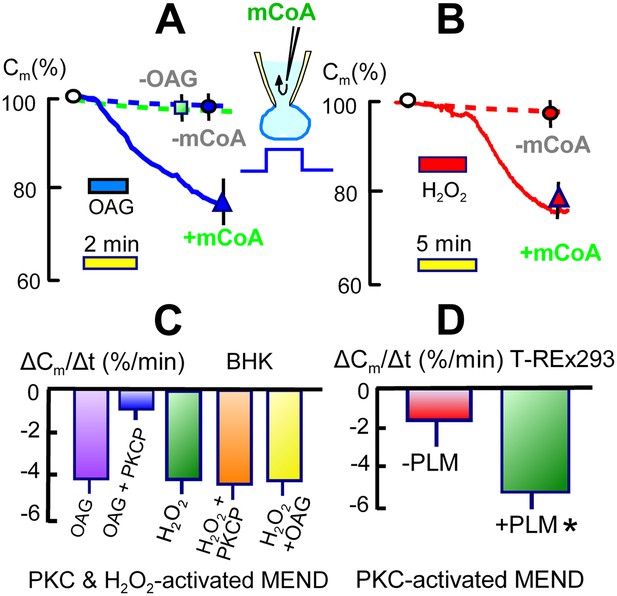
Activation of acyl CoA-dependent MEND without Ca transients.
(A) Pipette perfusion of mCoA (15 μM) without previously activating a Ca transient causes little or no endocytosis over 5 min (green square). Extracellular application of OAG (15 μM) for 1 to 2 min also has little or no effect, even after 6 min. With mCoA (15 μM) in the cytoplsamic solution, OAG initiates a decrease of membrane area that continues for several minutes after OAG is removed, amounting to 24% on average after 6 min. (B) Application of H2O2 (80 μM) for 4 min has little or no effect over 15 min in the absence of mCoA. With mCoA (15 μM) in the cytoplasmic solution, however, a large decrease of membrane area occurs when H2O2 is removed, amounting to 26% on average after 6 min. (C) MEND responses quantified as percent decrease of membrane area per min over 4 min. From left to right, the bar graphs quantify MEND caused by OAG, inhibition of OAG-induced MEND by PKC peptide 19–36 (1 μM in the pipette), MEND caused by H2O2, lack of effect of PKC peptide (19–35) on H2O2 MEND, and average MEND responses to H2O2 and OAG applied sequentially. (D) MEND in T-Rex-293 cells with inducible PLM expression. The bar graphs quantify the percent decrease of membrane area over 4 min after applying OAG. OAG-activated MEND is increased nearly three-fold when PLM expression has been induced for 24 hr.
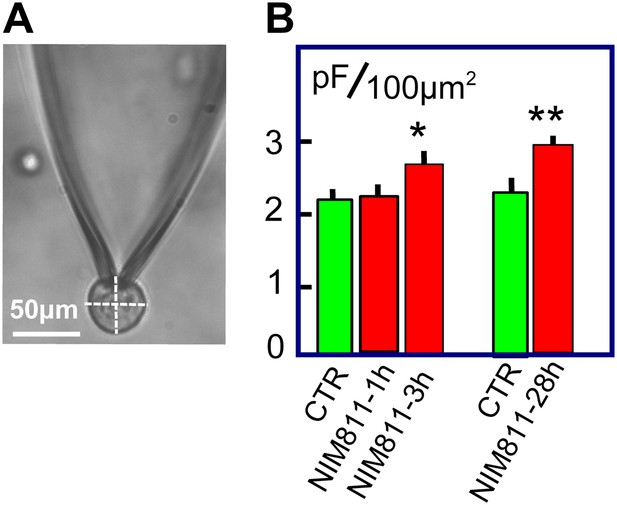
NIM811 increases surface area of cultured BHK cells.
(A) Micrograph of BHK cell during patch clamp. 25× LWD lens. Cell diameters were calculated as the average of a horizontal and vertical line transecting each cell, as indicated. The spherical area of each cell was calculated, and the ratio of the electrically determined area to the spherical area was calculated assuming 1pF to be 100 μm2. (B) First three bar graphs give results for BHK cells removed from dishes and incubated at 37°C for 1 or 3 hr without (CTR) and with NIM811 (2 μM). Results for control cells were not significantly different and were pooled. The fourth and fifth bar graphs give results for control cells (CTR) and cells grown with NIM811 (2 μM) for 30 hr.
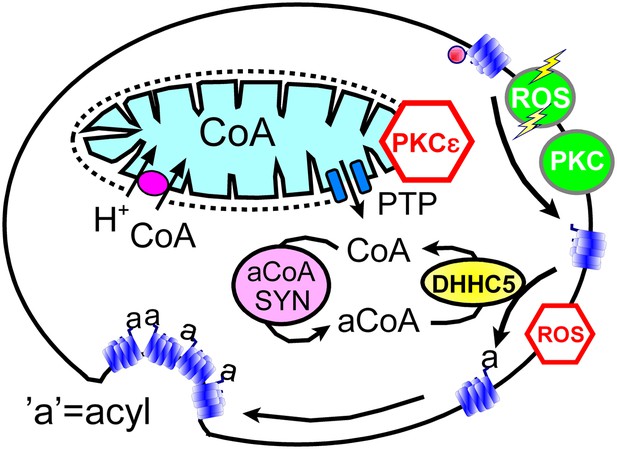
Hypothetical MEND pathway.
Mitochondria accumulate CoA via voltage-dependent transporters that are probably proton-coupled (Tahiliani, 1989). CoA can be released in response to PTP openings, either directly or more slowly via reverse CoA transport during mitochondrial depolarization. During a MEND response the activation of PKCs will restabilize mitochondria and promote MEND progression at the cell surface. PTP openings are inhibited when PKCs are activated ‘prior’ to the MEND protocol. Acyl CoA transients occur upon release of CoA because acyl CoA synthetases are CoA-limited (Idell-Wenger et al., 1978). We speculate that conventional PKCs and ‘transient’ oxidative stress increase the availability of palmitoylation sites at the surface membrane, whereas the immediate presence of ROS inhibits palmitoylation reactions.





