Massive palmitoylation-dependent endocytosis during reoxygenation of anoxic cardiac muscle
Figures
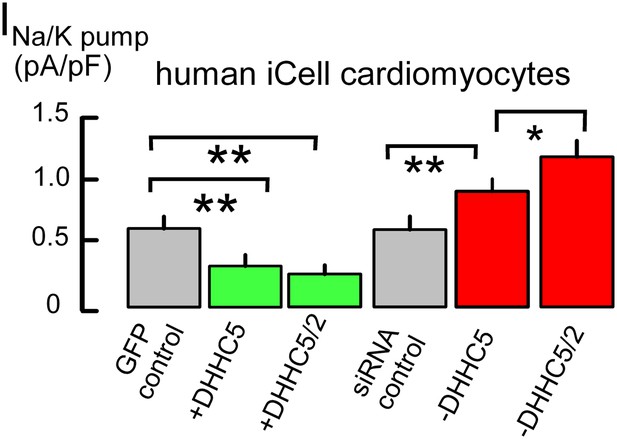
Na/K pump current densities are inversely dependent on DHHC5 expression in human iCell Cardiomyocytes.
From left to right, Na/K pump current densities in iCell Cardiomyocytes transfected with GFP to identify transfected cells, DHHC5, DHHC5 and DHHC2, transfected with scrambled siRNA, siRNA for DHHC5, and siRNA for DHHC5 and DHHC2. Na/K pump currents are decreased by 55% and 61% with DHHC5 and DHHC5/DHHC2 overexpression, respectively. Current densities are increased by 38% with DHHC5 knockdown and by 90% with DHHC5/DHHC2 knockdown, n >6 for all results.
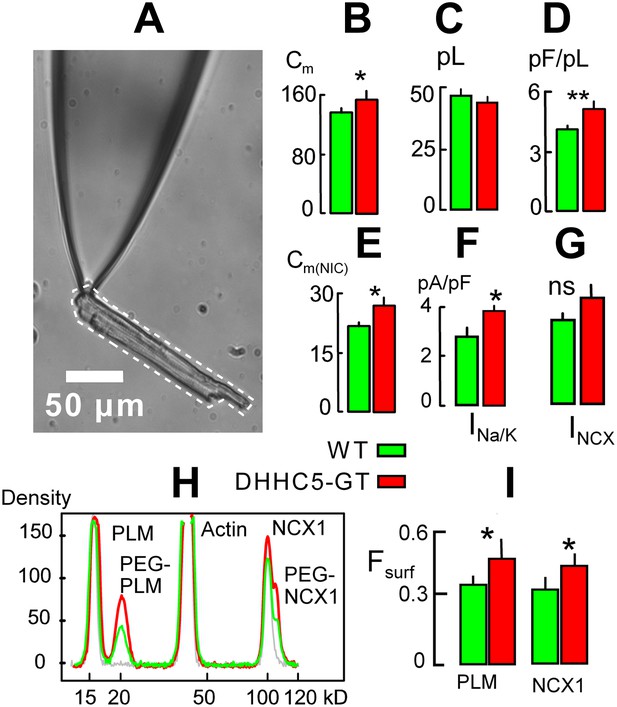
DHHC5-GT cardiac myocytes.
(A) Representative myocyte micrograph used to determine myocyte area/volume ratios. 25 × LWD lens. (B) Myocyte surface areas (Cm) are significantly increased in DHHC5-GT myocytes vs matched WT myocytes. (C) Myocyte volumes are not significantly different in DHHC5-GT myocytes from 4 to 6 week old mice. (D) Myocyte surface area/volume ratios are increased by 27% in DHHC5-GT myocytes. (E) Cardiac tissue Cm, monitored via NIC recording, is increased by 21% in right ventricular strips from DHHC5-GT mice (n = 5) vs control litter mates (n = 5). (F) Na/K pump current densities are increased by 32% in DHHC5-GT myocytes. (G) An average 17% increase of NCX1 current density is not significant. (H) Amine PEGylation assay to determine the surface membrane fractions of PLM and NCX1 in WT and DHHC5-GT hearts. Western blot density profiles are shown for three hearts, one control heart that was not PEGylated (gray), one WT heart (black) and one DHHC5-GT heart (red). PLM, actin and NCX1 are blotted, and the PEGylated PLM and NCX1 densities (i.e., protein resident in the cell surface) are shifted 5 kD from control densities. (I) Fractions of PLM and NCX1 that can be PEGylated, and therefore reside in the sarcolemma, are increased by 27 and 23%, respectively, in DHHC5-GT hearts. For both WT and DHHC5-GT hearts, n = 3. For all data from myocytes, n >40 using myocytes from three animals.
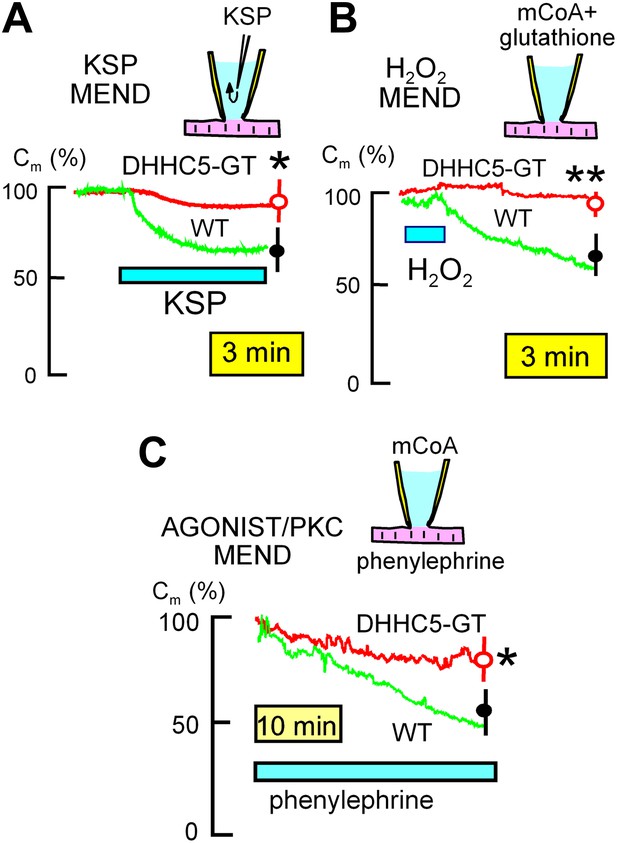
MEND evoked by three different stimuli is inhibited in DHHC5-deficient myocytes.
(A) KSP MEND. Cytoplasmic application of KSP solution via the patch pipette causes MEND responses in WT myocytes that take place with rough time constants of 2 min and that amount to 24% of the sarcolemma, on average. The KSP MEND responses are decreased by 74% on average in myocytes from DHHC5-GT animals. (B) H2O2 MEND. The application and removal of H2O2 (80 μM) results in 38% MEND responses, on average, with MEND occurring only after the oxidative stress is relieved. These MEND responses require that the cytoplasmic solution contains acyl CoA (mCoA, 15 μM). They are more reliable when glutathione (3 mM) is included in the cytoplasmic solution, presumably because final steps of endocytosis require a reducing environment. (C) GPCR MEND. MEND occurs slowly over 30 min in the presence of phenylephrine (30 μM) when the cytoplasm contains mCoA (20 μM). These endocytic responses, while slow, amount on average to >40% of the sarcolemma, and they are inhibited by 67% in myocytes from DHHC5-GT animals.
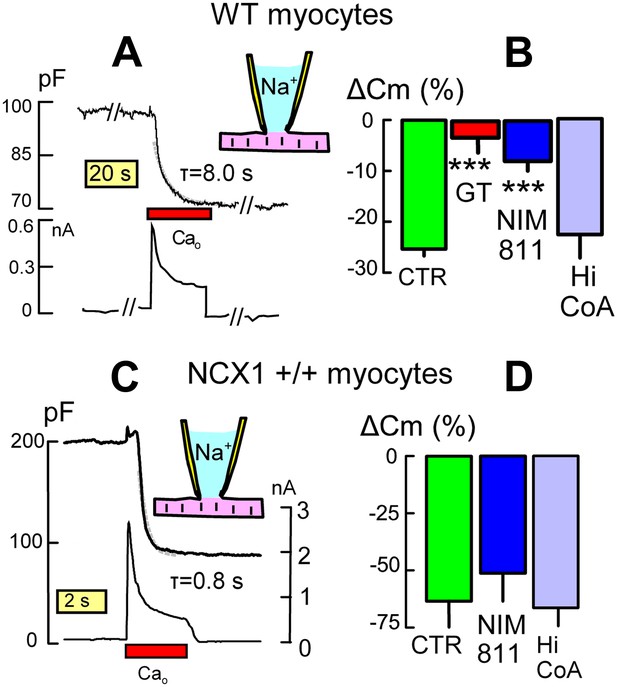
DHHC5 facilitates Ca-activated MEND in cardiac myocytes.
(A) Typical endocytic response caused by Ca influx (i.e., reverse Na/Ca exchange current) in a WT myocyte. Top record, Cm (i.e., membrane area); bottom record, membrane current. MEND occurs during Ca influx with a time constant of ∼8 s. (B) Ca-activated MEND in WT myocytes amounts to 27% of the sarcolemma on average. MEND is reduced by >80% in myocytes from DHHC5-GT animals and reduced 70% by pretreatment of cells with NIM811 (3 μM) for 1 hr. However, MEND is not reduced by inclusion of a high CoA concentration (4 mM) to acutely block acyl transferace activity. (C) Typical MEND response in a cardiac myocyte over-expressing NCX1 by 10-fold. Top trace, Cm (i.e., membrane area); bottom trace, membrane current. In these myocytes with four to sixfold larger Na/Ca exchange currents, Ca influx causes MEND that amounts to more than 50% of the sarcolemma in less than 2 s. (D) Large Ca transients overcome the dependence of MEND on palmitoylation. Ca-activated MEND in NCX1-overexpressing myocytes is unaffected by 1 hr treatment with NIM811 (3 μM) or by a high cytoplasmic CoA concentration (4 mM) to acutely inhibit palmitoylation.
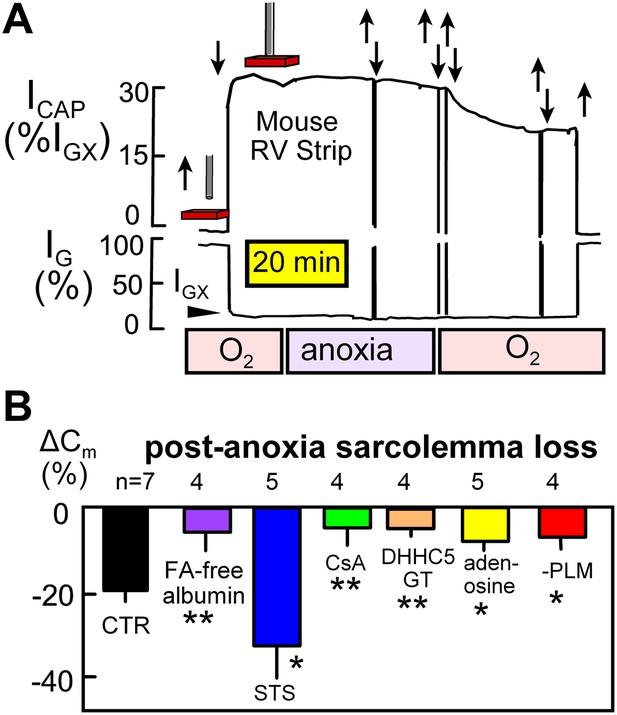
Electrical recordings of MEND during reoxygenation of anoxic cardiac muscle.
(A) Noninvasive Cm (NIC) recording in superfused right ventricular strips. The capacitive signal (ICAP), reflecting sarcolemmal area in a hemisphere of tissue beneath the electrode, is stable during 30 min periods of anoxia, but the NIC signal decreases on average by 21% over 25 min upon reoxygenation of the tissue. (B) Loss of sarcolemma during reoxygenation for 25 min after a 30 min period of anoxia. The first bar graph quantifies the composite response for control (WT) muscles. Subsequent bars document from left to right that MEND is strongly inhibited by perfusing hearts for 15 min with FA free albumin (60 μM) before isolating muscle strips, is strongly enhanced by a low concentration (0.1 μM) of staurosporine, is potently inhibited by cyclosporine (CyS) (1 μM), is strongly reduced in muscles from DHHC5-deficient mice, is strongly reduced by the ‘preconditioning’ hormone, adenosine (100 μM), and is strongly reduced in cardiac muscle lacking PLM.
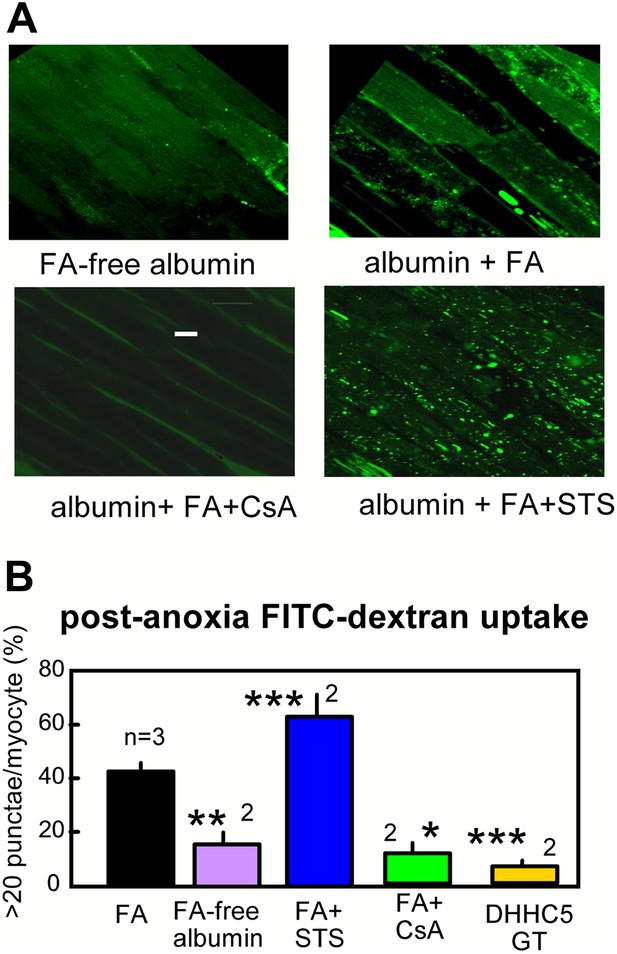
Optical recordings of MEND during reoxygenation of anoxic cardiac muscle.
(A) Micrographs of FITC-dextran uptake in arterially perfused mouse hearts after 30 min of anoxia, followed by 25 min reoxygenation with perfusion of FITC-dextran (0.5 mM) and a 10 min washout at 25°C. Confocal images are from myocytes that are a few cell layers below the outer left ventricular cardiac surface. Scale bar, 30 μm. (B) Composite results for hearts with control perfusate (i.e., 0.1 mM albumin with 0.1 mM FA), with perfusate containing 0.1 mM FA-free albumin, with control perfusate containing cyclosporine (CsA, 2 μM), with control perfusate containing staurosporine (STS, 0.1 μM), and DHHC5-GT hearts with normal perfusate.
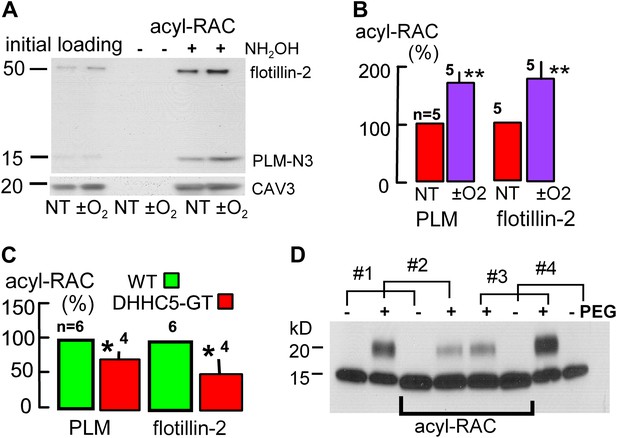
Palmitoylation is increased during reoxygenation-induced cardiac MEND.
Palmitoylation of PLM and flottilin-2 during reoxygenation of anoxic cardiac tissue. (A) Experiments were performed in a pairwise fashion. One heart was quick-frozen after perfusion with oxygen for 45 min (not treated, NT) and one after being subjected to the anoxia/reoxygenation protocol, followed by biochemical analysis of palmitoylation. Left lanes show loading controls from initial lysates; right four lanes show samples after deacylation and precipitation with cysteine-reactive beads. No protein is detected without deacylation. Caveolin-3 was blotted on the same gel after stripping secondary antibodies. (B) Five paired data sets from experiments as in ‘A’, with palmitoylation calculated relative to palmitoylated caveolin-3 densities. (C) Constitutive palmitoylation of PLM and flotillin-2 in DHHC5-GT hearts, not subjected to anoxia/reoxygenation, is decreased 27 and 51%, respectively. Palmitoylation is not clearly changed in hearts subjected to 30 min anoxia without reoxygenation (‘−O2’). (D) Combined measurements of the surface membrane fraction and the palmitoylated fraction of PLM. Hearts #1 and #4 were not PEGylated; hearts #2 and #3 were PEGylated with PEGylated PLM fractions amounting to 40 and 46%, respectively, in the initial lysate. In the acyl-RAC pull-down of palmitoylated PLM, the surface membrane (PEGylated) fractions of PLM amount to only 17 and 23% in hearts #2 and #3, respectively. Thus, surface membrane PLM is substantially less palmitoylated than internalized PLM.
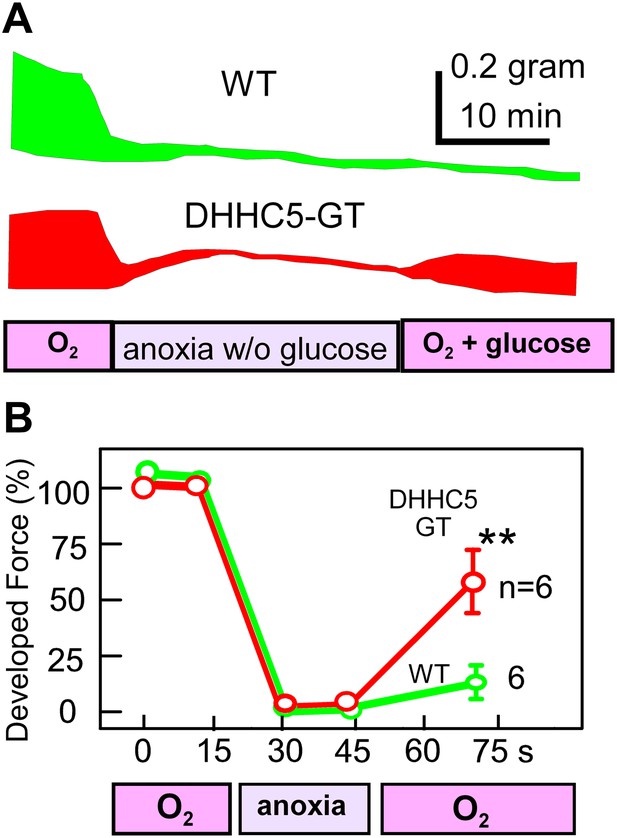
MEND correlates with impaired functional recovery of cardiac muscle from anoxia.
Contractile function of mouse right ventricular strips, paced at 0.25 Hz, during anoxia for 30 min without glucose, followed by reoxygenation with glucose (15 mM) for 25 min. (A) Representative force envelopes of right ventricular strips. (B) Composite results for right ventricular strips from WT and DHHC5-GT mice. For both sets, n = 6.
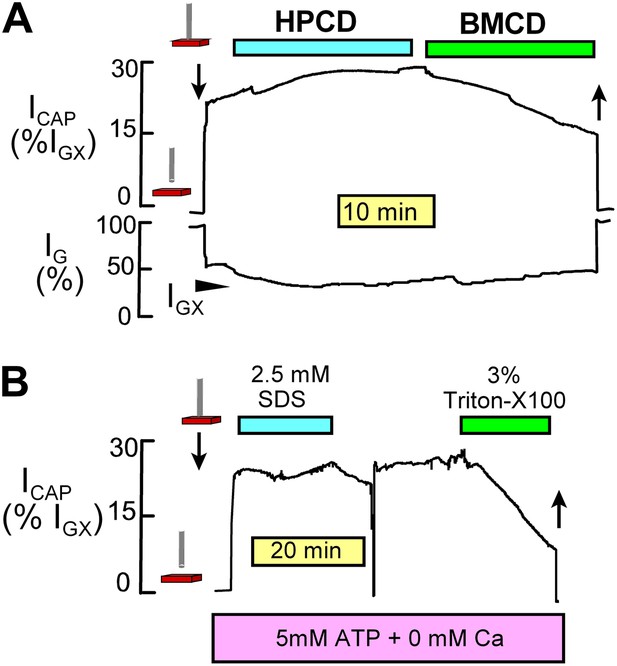
NIC recording monitors sarcolemmal area and is insensitive to sarcolemmal conductance changes.
(A) As described previously (Lariccia et al., 2011), (2-hydoxypropyl)-β-cyclodextrin (HPCD) extracts cholesterol from fibroblasts without causing Cm changes. However, methyl-β-cylodextrin (BMCD) causes large decreases of Cm that presumably reflect extraction of both phospholipids and cholesterol. Similarly, NIC signals from right ventricular strips are unaffected by application of HPCD (8 mM) but are strongly decreased by application of BMCD (8 mM). (B) Muscle incubated with ATP and 0 Ca with 1 mM EGTA to avoid contraction when the sarcolemma develops ruptures. A rather high concentration of sodium dodecylsulfate (SDS, 2.5 mM) does not significantly decrease NIC signals. Although this concentration of SDS generates sarcolemmal leaks with great certainty, it does not effectively extract the sarcolemma from the intact muscle preparation. By contrast, Triton X100 at 3% very effectively decreases NIC signals from the same muscle, as expected for an effective membrane extraction. These results verify that NIC signals are not affected by increases of sarcolemmal conductance until cellular conductance becomes significant with respect to that of the extracellular space.





