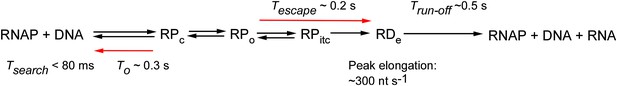
Single-molecule tracking of the transcription cycle by sub-second RNA detection
Figures
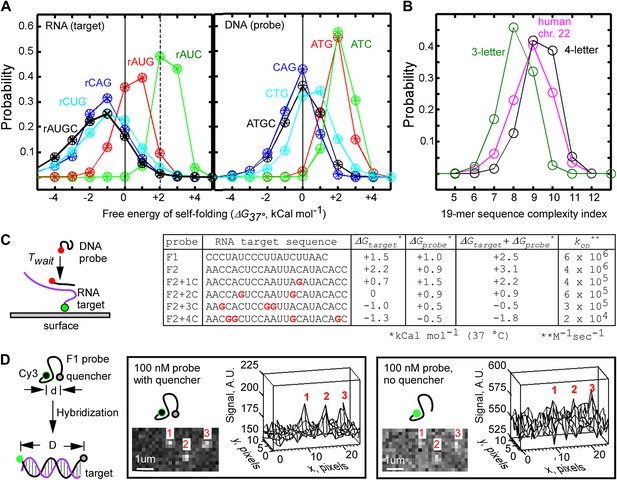
Design of fastFISH probe-target pairs.
(A) Probability distribution of Mfold-calculated free energies of self-folding of randomly selected, single-stranded 19-mer RNA (left) and DNA (right) oligonucleotides, composed of three or four bases. Results of analysis of three independent sets are shown as ‘+’, ‘x’, and ‘○’. About 100,000 three-letter sequences, and about 300,000 four-letter sequences were analyzed in each set. (B) Lempel–Ziv complexity analysis of three-letter 19-mer oligonucleotides (one set of ∼100,000 AUC sequences), four-letter 19-mer oligonucleotides (one set of ∼300,000 AUGC sequences), and all tiling 19-mers from the exome of the human chromosome 22. (C) Single-molecule measurements of hybridization rates of fastFISH probe-target pairs, and the effect of G-residues in the targets on the rates. Left: schematic of experiment. Cy3-labeled 90-base RNA oligonucleotides containing a single target sequence were immobilized on a glass surface through a biotin moiety at the 3′ end. Atto633-labeled DNA probes were injected into the imaging flow cell, and their hybridization was detected using TIRF/CoSMoS to obtain the probe arrival time Twait. Right: table of RNA target sequences, Mfold-calculated free energies of self-folding of RNA targets (ΔGtarget), DNA probes (ΔGprobe), combined energies of targets and probes, and on-rates calculated based on probe Twait and concentrations. (D) Self-quenching approach to reduce fluorescence background from unbound DNA probes in TIRF imaging of probe-target hybridization. Left: schematic of experiment. A quencher (e.g., Iowa Black FQ) is placed on one end of a DNA probe, and a fluorophore (e.g., Cy3) is placed on the opposite end of the DNA probe. The short persistence length of single-stranded DNA (lo ∼0.8 nm, Smith et al., 1996; Dessinges et al., 2002) enables quenching of Cy3. Upon hybridization to the target, the distance between Cy3 and Iowa Black FQ increases due to the larger lo ∼50 nm of the DNA-RNA duplex, leading to an increase of Cy3 fluorescence. Middle: representative TIRF image and a corresponding three-dimensional plot of target-hybridized F1 probes acquired in the presence of unbound, self-quenched F1 probe at 100 nM. Right: same set of molecules imaged in the presence of unbound, unquenched F1 probe at 100 nM.
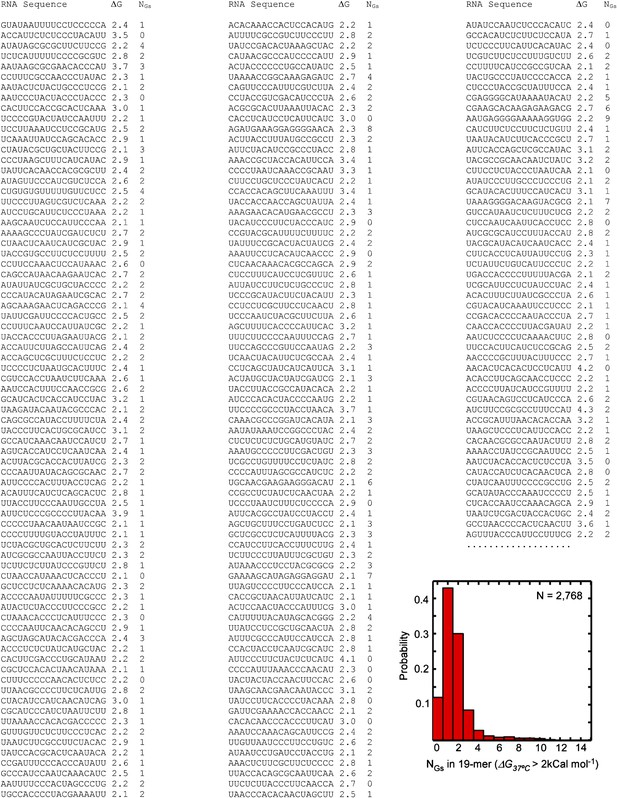
Sample sequences of the unstructured 19-mer RNA oligos.
Representative sequences of randomly picked 19-mer 4-base RNA oligonucleotides with free energy of self-hybridization ΔG37°C of 2 kCal mol-1 or higher (2,768 sequences total, selected from a pool of 339,612 randomly picked sequences) are shown. First column: RNA sequence; second column: Mfold-calculated ΔG37°C; third column: number of G residues in the sequence. The histogram is the probability distribution of the number of G residues per sequence.
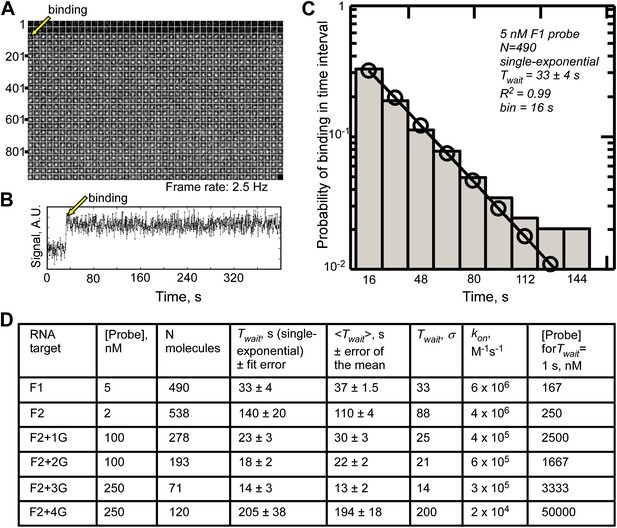
Single-molecule measurements of fastFISH probe-target hybridization rates using TIRF/CoSMoS.
(A) Representative real-time video montage of binding of an F1 probe molecule to a location in which an F1 target molecule has been previously registered (region of interest 5 × 5 pixels). The video frame in which binding of F1 probe occurred is shown with an arrow. (B) Fluorescence time trace corresponding to the montage in Panel A. (C) Probability distribution of F1 probe binding times fit to a single exponential function to determine the mean binding time Twait. (D) Summary of single-molecule measurements of probe-target hybridization rates for probes F1 and F2, and F2 target variants containing 1, 2, 3, and 4 G residues, at the probe concentrations shown. Mean binding time Twait was estimated by fitting the probability distributions to a single exponential function. Goodness of fitting is evident from the consistency among the values of Twait, <Twait>, and the standard deviation of dwell times, σ. The last column shows the calculated concentrations of respective probes required to achieve mean Twait of 1 s in a real-time nascent RNA detection experiment. Concentrations of self-quenched probes above 1000 nM, and unquenched probes above 100 nM, are generally incompatible with single-molecule imaging. Fit error for Twait is given for 95% confidence intervals, and error of the mean was calculated as σ/√(Nmolecules).
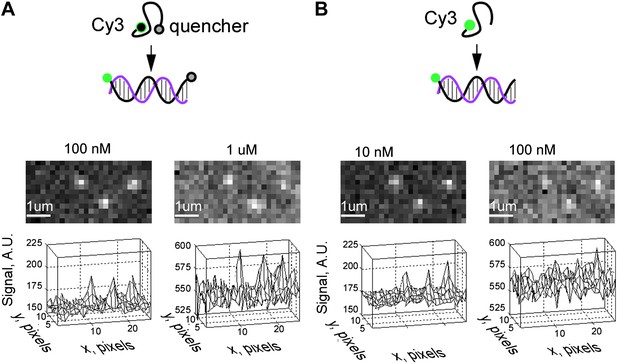
Reduction of fluorescence background of free fastFISH probes by self-quenching: additional representative images.
(A) Top: self-quenched F1 probe (Cy3 dye at the 5′ end and Iowa Black FQ quencher at the 3′ end). Middle: representative images of signals from self-quenched F1 probe molecules hybridized to surface-immobilized RNA targets, in the presence of free Cy3-F1-FQ probe at 100 nM and 1 μM concentrations. Bottom: same images shown as three-dimensional plots. (B) Same set of molecules as in (A), but in the presence of free Cy3-F1 containing no quencher at 10 nM and 100 nM concentrations.
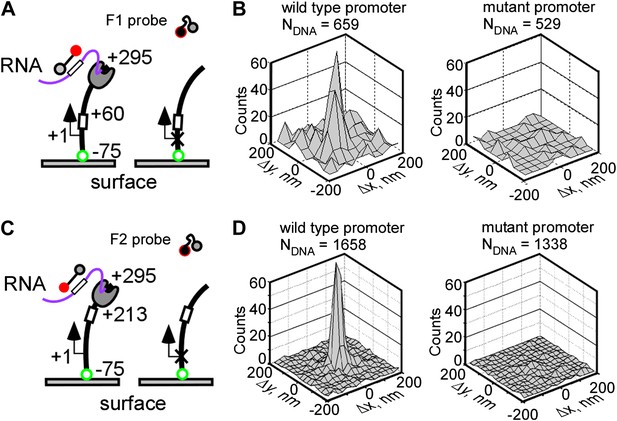
Real-time single-molecule detection of transcription by T7 RNAP using fastFISH.
(A) Schematic of experiment. DNA templates containing a single consensus promoter for T7 RNAP (+1), or a single null mutant promoter (cross), were immobilized on a surface, with the promoter directing transcription towards the free end (+295). The templates contained the F1 fastFISH target sequence downstream from the promoter (from +28 to +46) which was expected to become available for hybridization in the nascent RNA after the RNAP active site reaches position +60. (B) Co-localization analysis of F1 probe-DNA interactions in a representative experiment. Left: wild type promoter. Right: mutant promoter. (C) and (D) Same as panels (A) and (B), but for the F2 target and probe. The templates contained the F2 fastFISH target sequence (from +181 to +199) which was expected to become available for hybridization in the nascent RNA after the RNAP active site reaches position +213.
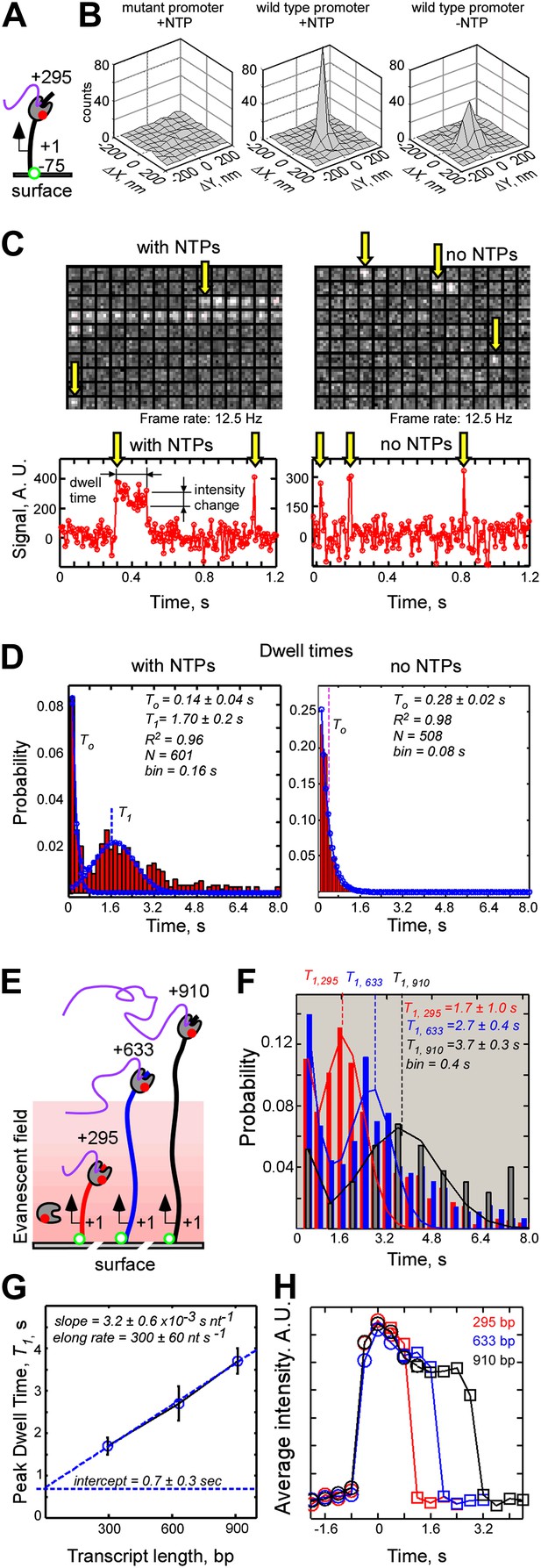
Single-molecule dynamics of T7 RNAP-DNA interactions.
(A) Schematic of experiment. (B) Co-localization analysis of RNAP-DNA interactions. Left: null promoter DNA template in the presence of NTPs. Center: wild type promoter DNA template in the presence of NTPs. Right: wild type promoter DNA template in the absence of NTPs. (C) Representative data. Top: video montages of RNAP interactions with template containing consensus promoter for a 5 × 5 pixel region of interest centered at a single, photobleached DNA molecule (1 pixel = 200 nm, imaged at 12.5 Hz). Bottom: fluorescence time traces corresponding to the montages shown on top. Baseline of zero intensity indicates no binding. Left: experiment carried out in the presence of NTPs. Right: experiment carried out in the absence of NTPs. Yellow arrows indicate the first frames of RNAP binding events. (D) Dwell time probability distributions of RNAP-DNA binding events. Left: experiment carried out in the presence of NTP. Fitting to a sum of single exponential and Gaussian functions is shown in blue. Right: experiment carried out in the absence of NTPs. Fitting to a single exponential function is shown in blue. (E) Dependence of the peak dwell time of RNAP-DNA interactions on the length of the transcribed DNA segment: schematic of experiment. DNA templates containing transcribed segments spanning from +1 to +295 (red), +633 (blue), or +910 (black) were separately immobilized, and interactions of labeled RNAP were recorded at 2.5 Hz. (F) Dwell time probability distributions of RNAP-DNA interactions for the three DNA templates shown in (E). N = 749 for the +1…+295 template (red), N = 716 for the +1 … +633 template (blue), and N = 213 for the +1 … 910 DNA template (black). The peak dwell times were calculated by fitting the distributions to a sum of single exponential and Gaussian functions. (G) Plot of the peak dwell time of RNAP-DNA interactions vs the length of the transcribed DNA segment. (H) Decay of intensity of RNAP fluorescence signal during productive RNAP-DNA interactions as an indicator of elongation by RNAP. All RNAP-DNA interactions having dwell times longer than 0.8 s (experiments in F) were post-synchronized by RNAP binding (t = 0, circles) and by RNAP run-off (squares), and weight-averaged plots of RNAP binding and run-off were plotted for DNA templates having transcribed segments of different lengths (red − 295 bp; blue − 633 bp; black − 910 bp). Time offsets between RNAP binding and run-off were set at peak dwell lifetimes, T1, for the respective DNA templates measured in (F).
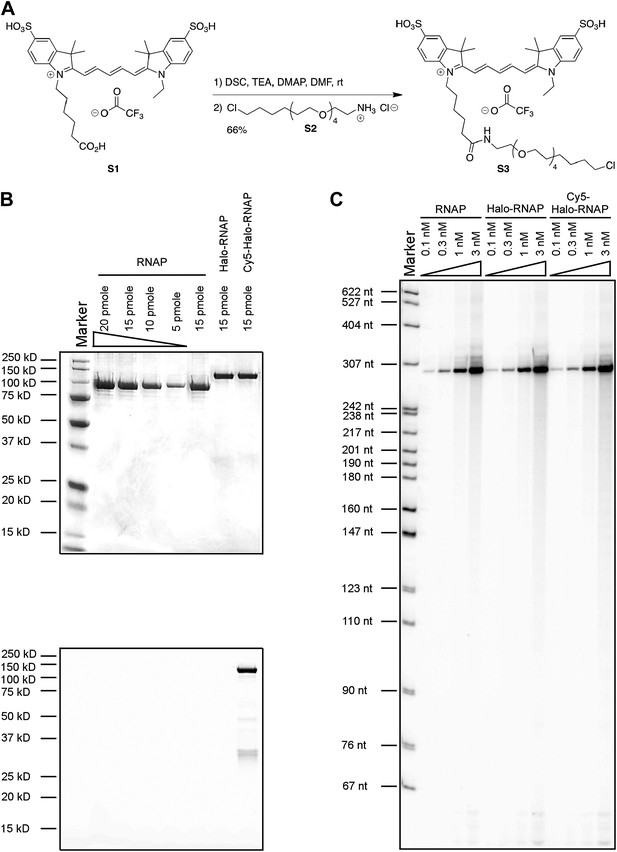
Labeling and biochemical characterization of T7 RNAP activity.
(A) Synthesis of Cy5-conjugated HaloTag Ligand (S3) used to label RNAP from Cy5 acid S1 and HaloTag ligand-amine S2. See ‘Materials and methods’ for details. (B) SDS-PAGE gel images of the unlabeled (‘RNAP’: no HaloTag; ‘Halo-RNAP’: with HaloTag) and the Cy5-labeled Halo-RNAP (‘Cy5-Halo-RNAP’) T7 RNAP. All RNAPs have a 6(His) tag at the N-terminus used for purification. Top: image stained with Coomassie Brilliant Blue. Bottom: the same gel scanned for Cy5 fluorescence (prior to Coomassie staining). (C) In vitro transcription activity of RNAP. The concentrations of RNAP derivatives were as indicated. The DNA template (a PCR fragment spanning from −75 to +295 of the concensus T7 RNAP promoter), was used at 10 nM. RNA products were labeled by incorporation of α32P-ATP, resolved on a denaturing polyacrylamide gel, and imaged by autoradiography. The major bands at ∼295 nt are the expected run-off products.
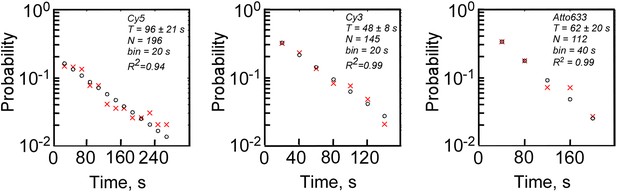
Measurements of photobleaching times of fluorophores used in this study.
Double-stranded, biotinylated, labeled DNA molecules were immobilized on a passivated glass surface, imaged with TIRF microscope in the presence of oxygen scavengers, and photobleaching time-traces of fluorophores were recorded at 2.5 Hz. The same laser intensities were used as in single-molecule transcription experiments (532 nm at 300 W cm−2 to excite Cy3, and 640 nm at 100 W cm−2 to excite Cy5 and Atto633). The times of photobleaching of individual molecules were binned, plotted as probability histograms (red crosses at bin centers), and the histograms were fit to single exponential function (black circles at bin centers) to estimate mean photobleaching lifetimes (T).
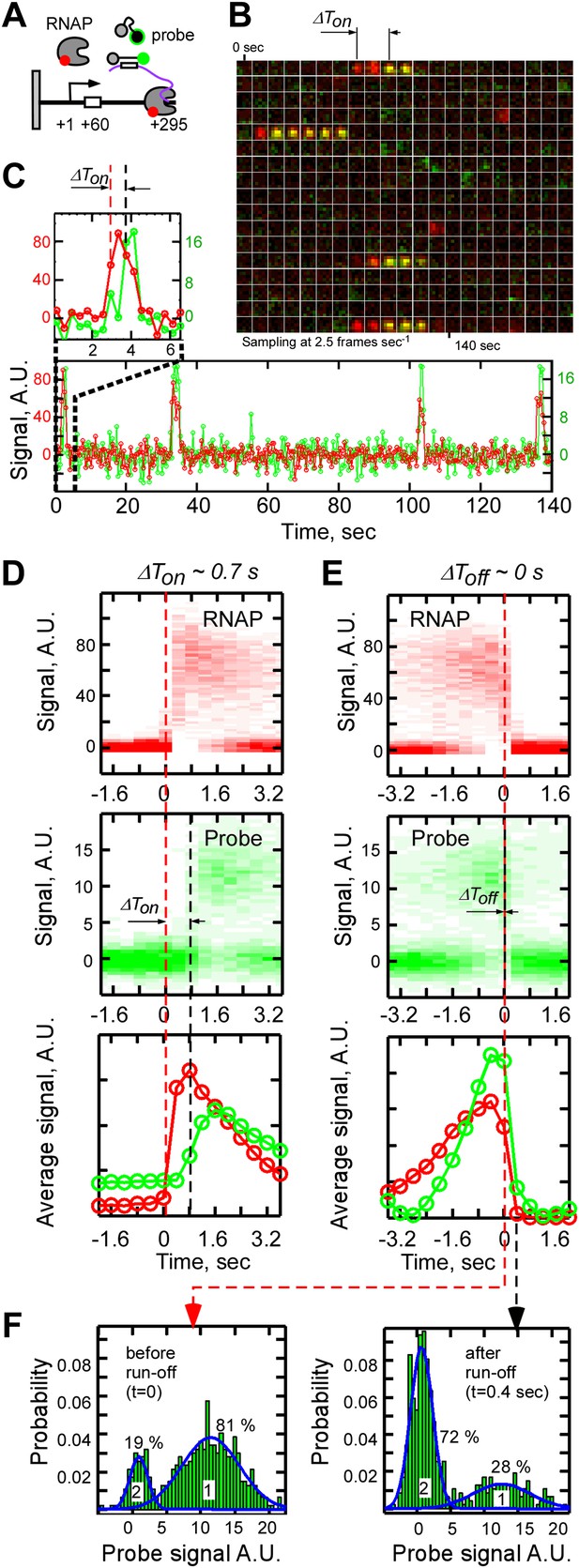
Single-molecule dissection of the T7 RNAP transcription cycle using fastFISH.
(A) Schematic of experiment. (B) Merged video montages (5 × 5 pixel region of interest) centered at a single, previously photobleached DNA molecule. Interactions of RNAP (false colored in red) and probe (false colored in green) with the DNA locus were imaged simultaneously at 2.5 Hz. Four representative transcriptional events are shown. (C) Fluorescence time traces corresponding to the montage shown in Panel B (RNAP and probe are shown in red and green, respectively). The first transcriptional event is zoomed in and shown on top. (D) Calculation of the time delay of probe binding with respect to RNAP binding (ΔTon) from heat maps of all RNAP-DNA (top) and all probe-DNA binding events (middle) post-synchronized by RNAP binding (Ton, NDNA = 112, Nevents = 469). Baseline of zero intensity indicates no RNAP/probe binding. Time point t = 0 corresponds to the frame immediately before RNAP binding (red dashed line). On the bottom are the weight-averaged, normalized signal intensities of RNAP and probe binding calculated based on the heat-maps of all RNAP-DNA (red) and probe-DNA (green) binding events. (E) Calculation of the time delay of probe dissociation with respect to RNAP run-off (ΔToff) from heat maps of all RNAP-DNA and probe-DNA dissociation events post-synchronized by RNAP dissociation (Toff). Time point t = 0 corresponds to the frame immediately before RNAP dissociation (red dashed line). (F) Calculation of the efficiency of real-time RNA detection (left), and of the fraction of events in which RNA was released from DNA upon RNAP run-off (right). Productive RNAP-DNA interactions were post-synchronized by RNAP dissociation as shown in Panel E, and probability distribution of probe signal intensity was plotted for the time point immediately before RNAP run-off (left, t = 0, for the efficiency of RNA detection) and the time point immediately after RNAP run-off (right, t = 0.4 s, for the fraction of RNA released upon run-off). Fits of distributions to sums of two Gaussian functions are shown in blue (R2 = 0.92). The higher mean-value Gaussian (peak 1) corresponds to the events with the probe signal present and the lower mean value (peak 2) corresponds to the events without the probe signal present.
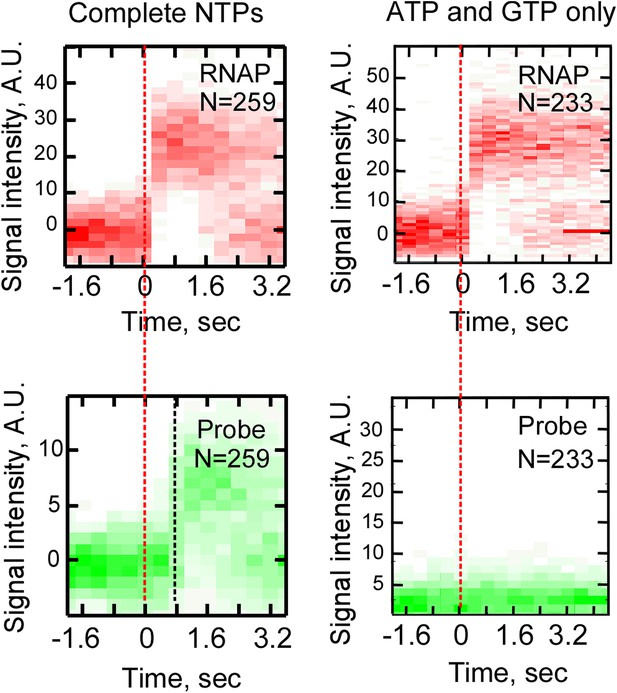
Control single-molecule transcription experiments in the presence of an incomplete set of NTPs.
Cy5-labeled RNAP and Cy3-labeled F1 probe were used in a reaction at the presence of a full set of NTPs (ATP, GTP, UTP, and CTP, 0.5 mM each, left), or ATP and GTP only (1 mM each, right). Top row: heat map of all RNAP-DNA interactions post-synchronized by RNAP binding (red dashed line). Bottom row: heat map of all F1 probe-DNA interactions post-synchronized by RNAP binding.
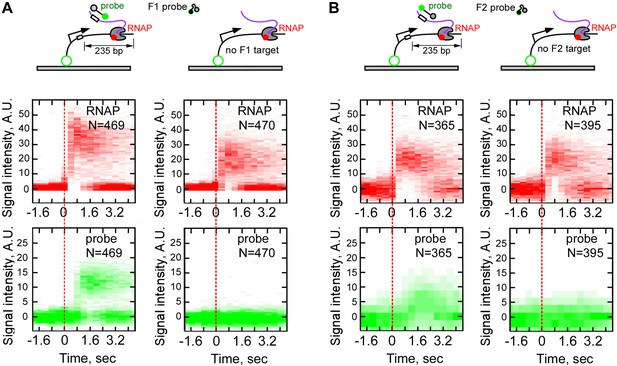
Sequence-specificity control of real-time RNA detection by fastFISH.
(A) Sequence-specificity of probe F1. Top row: schematic of experiments with DNA templates containing the sequence of F1 target (left) and DNA templates lacking the sequence of F1 target (right). Middle row: heat map of all RNAP-DNA interactions post-synchronized by RNAP binding (red dashed line). Bottom row: heat map of all F1 probe-DNA interactions post-synchronized by RNAP binding. Left panels show the same dataset as shown in Figure 3 for side-by-side comparison. (B) Same as panel (A), but for probe F2 (the probe signal was weaker, because lower laser power was used in the experiment shown).
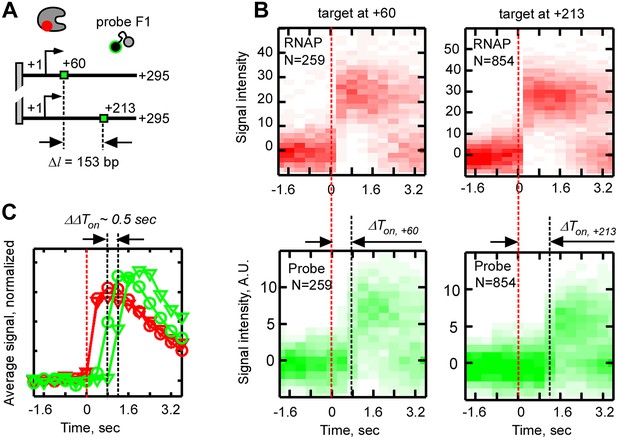
Effect of the distance between the promoter and the probe target sequence on the time delay between probe and RNAP binding, ΔTon.
(A) Schematic of the experiment. The F1 target sequence was inserted at two different positions with respect to the promoter: +28 (top template) and +181 (bottom template). The numbers ‘+60’ and ‘+213’ indicate the expected positions in the respective templates which the RNAP active site needs to reach before the probe target sequence in the nascent RNA becomes available for hybridization. (B) Calculation of the time delay between probe and RNAP binding (ΔTon) from heat maps of all RNAP-DNA (top) and all probe-DNA binding events (bottom) post-synchronized by RNAP binding. Data for the ‘+60’ and ‘+213’ templates are shown on the left and right, respectively. (C) Calculation of the difference in the time delay of probe binding (ΔΔTon) from weight-averaged signal profiles of RNAP (red) and probe binding (green). Profiles for the +60 template are shown as circles, and profiles the +213 template are shown as triangles.