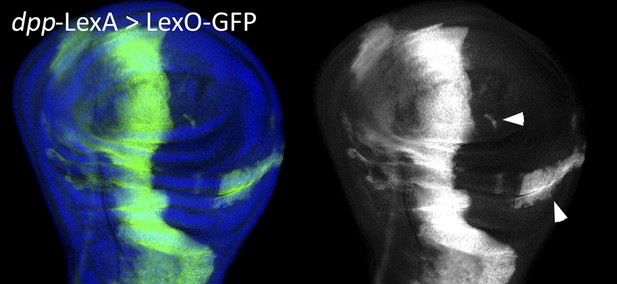
Transgressions of compartment boundaries and cell reprogramming during regeneration in Drosophila
Figures
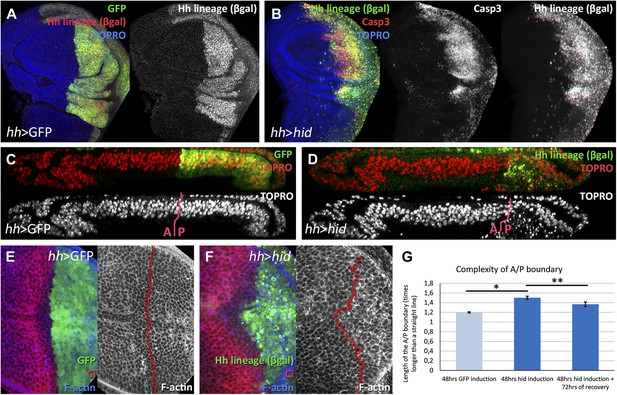
Ablation of the posterior compartment.
Wing imaginal discs after 48 hr of induction of GFP (A, C and E, controls) or hid (B, D and F). Note that in both cases the βgal lineage label is acquired by all the posterior cells (A and B). The disc in B shows high levels of apoptosis, indicated by Caspase3 activity. (C and D) Cross-sections of wing discs perpendicular to the A/P border at the level of the wing pouch. The peripodial membrane is on top, the columnar epithelium on the bottom. Anterior compartment at the left, posterior at the right. There is a marked reduction of the size of P compartment in which hid is expressed (D). The epithelium is much thinner than in control disc (C), indicating a big reduction of cell number due to the ablation (compare the number of nuclei in the right part of C and D). (E–G) Shape of the A/P border after 48 hr of GFP (E) or hid (F) induction. In the later this border becomes wiggly and inter-digitized, a feature quantified in panel G. By allowing 72 hr of recovery this effect is partially recovered (third bar in panel G). Bars represent S.E.M., n>15 in each genotype and time point, *p<0.01, **p<0.05. See also Figure 1—figure supplement 1.
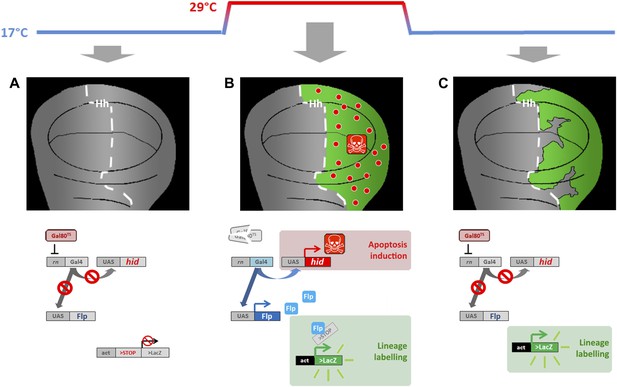
Genetic ablation and lineage labeling system.
(A) In discs from larvae of the genotype indicated maintained at 17°C the Gal80TS repressor blocks Gal4 activity, thus the UAS vectors cannot be activated. (B) By rising the temperature to 29°C the Gal80TS is inactivated, thus provoking two effects: (i) over-expression of the pro-apoptotic vector UAS-hid, causing massive cell death in the Hh domain (P compartment), (ii) over-expression of the UAS-Flipase construct, which in turn induces recombination in the act>stop>LacZ cassette. This recombination labels indelibly all the cells of the posterior compartment (green label in the diagram). (C) By taking back the larvae to 17°C the Gal80TS activity is recovered, thus blocking the expression of both hid and Flp. This allows the recovery of the ablated domain. The lineage labeling also allows recognizing if cells originated outside the ablated domain (not labeled) are participating in regeneration.
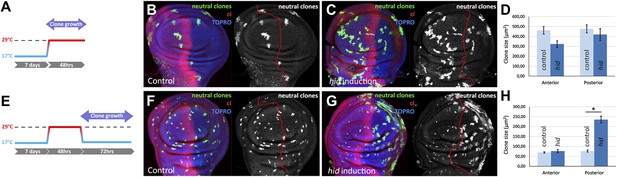
Clonal analysis of growth in discs in which the P compartment has been ablated.
(A–D) GFP-labeled clones only allowed growing during the ablation period. The clones were induced at the beginning of the ablation and the discs fixed at the end of it. Control disc (B) a hid-induced disc (C) and clone size quantification (D) are shown. Note the absence of major differences in clone size between control discs and hid-induced in both compartments. (E–H) Clones induced at the end of the ablation period and scored after 72 hr of recovery period at 17°C (see diagram E). Again, a control disc (F), a hid-induced disc (G), and quantification (H) are shown. Note in G that the clones in the P compartment are much bigger than those in the A compartment of the ablated discs and those of the P compartment in controls (F). Bars represent S.E.M., n>25 in each genotype and time point, *p<0.001.
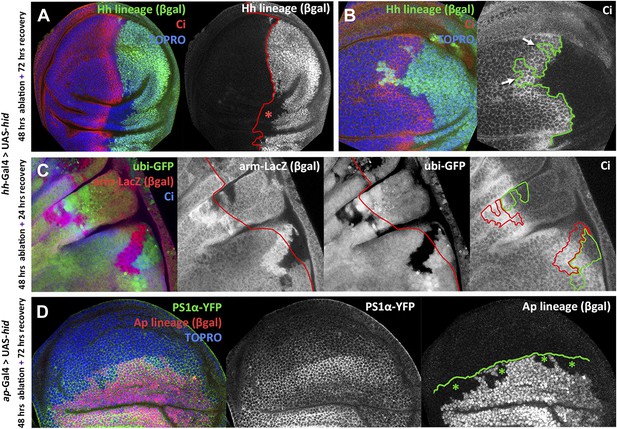
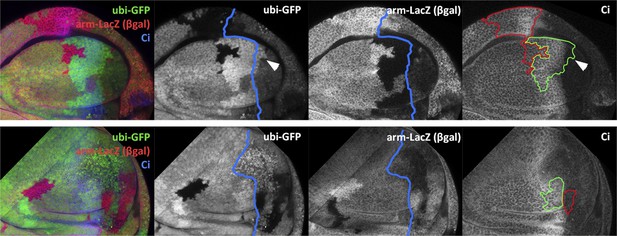
Transgressions of the A/P and the D/V border caused by ablation of the P or the D compartment.
(A and B) Wing discs after 48 hr of Hid treatment followed by 72 hr of recovery. The original Hh lineage is labeled by ßgal (green), the A compartment is marked with an anti-Ci antibody (red) and the nuclei with Topro (blue). (A) Note the presence of groups of cells (asterisk) originated in the anterior compartment (they lack the βgal label) but that have lost Ci activity, indicating that they have lost anterior identity. (B) The unexpected finding that cells from the P compartment–they are part of the Hh lineage–can penetrate in the A compartment and to acquire anterior identity, as demonstrated by the Ci marker (arrows) (C) Portion of a disc in which the P compartment has been ablated, containing two sets of ‘twin’ clones labeled with GFP-green/LacZ ßgal (see ‘Materials and methods’ for details). The clones were initiated at the beginning of the ablation of the P compartment and fixed 24 hr after the end of ablation. In those panels the A/P borderline is blue and the twin clones delineated in red or green. Note that the two cases the clones cross over the A/P line. (D) Wing disc after 48 hr of Hid induction in the dorsal compartment followed by 72 additional hours of recovery. Note the presence of groups of cells (asterisks) that in spite of their ventral origin (lack of dorsal lineage βgal label) now present dorsal markers as the mew Integrin (PS1α). See also Figure 3—figure supplements 1 and 2.
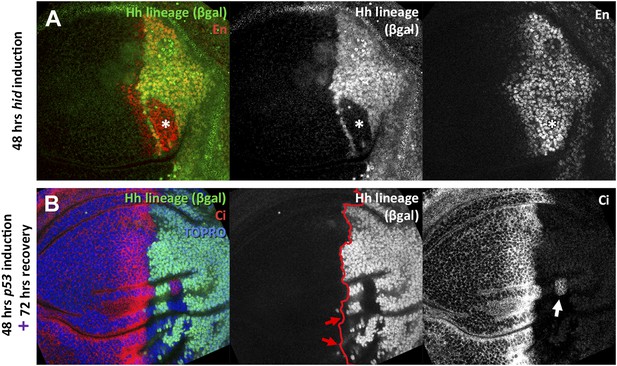
Transgressions detected during the ablation period and transgressions using p53 as apoptotic inducer.
(A) Wing disc fixed at the end of 48 hours hid induction. Note that the trespassing cells (with lack of βgal label, indicated by an asterisk), have activity of the posterior marker engrailed. (B) Wing disc after 48 hr of p53 over-expression in the posterior compartment and 72 additional hours of recovery. Note the presence of compartment transgressions (red arrows, cells that lack the βgal label). Occasionally, part of the cells of a transgression retain its original identity, as the example pointed with a white arrow, in which a group of Ci positive cells remain trapped in the posterior compartment.
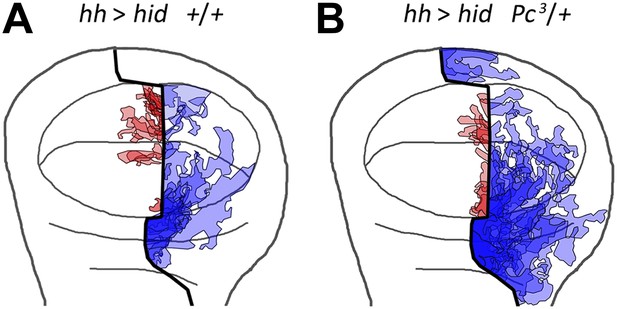
Localization on the wing disc of the different transgressions of the A/P border.
We have plotted all the transgressions found in after overexpressing the pro-apoptotic gene hid a disc containing the normal doses of Polycomb (A) or only one dose (B). The transgressions from A to P are in light blue and the P to A in light red. The darker color indicates the superimposition of several transgressions in some regions. Note that virtually all the transgressions reach the A/P border. Those from A to P exhibit some preference for the region between hinge and pouch, whereas those from P to A localize to the wing pouch. The overall preferential location of the transgressions near the A/P border rules out the possibility of incomplete label of the hh lineage.
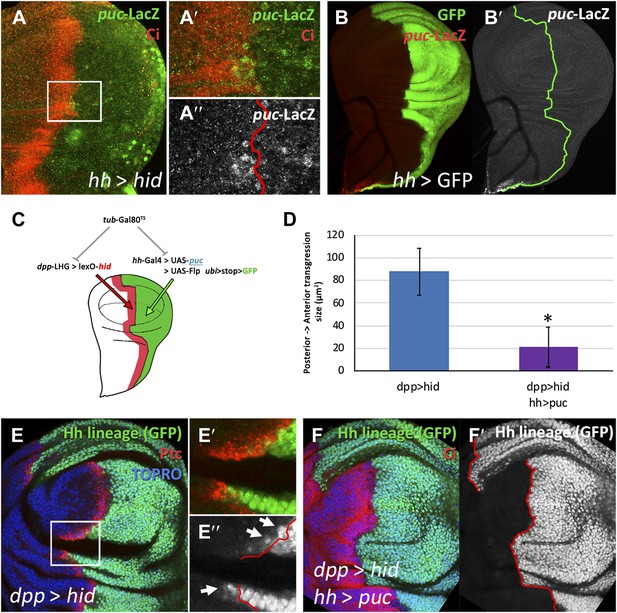
Involvement of the JNK pathway in the transgression of the A/P boundary during regeneration.
(A–A′′) Wing disc and magnification showing activation of puc-LacZ (green) after ablation of the P compartment. The A/P boundary is outlined by Ci (red) label. Note that some anterior cells contain LacZ expression. The puc–lacZ staining is not particularly strong in the P compartment because it is located predominantly on the basal side of the disc, where the dying cells accumulate. (B and B′) Control non-ablated disc showing absence of puc-LacZ activity, except in the stalk region where it is normally expressed. The panel C illustrates the experiment designed to test the requirement for JNK activity for crossing the A/P boundary during regeneration. After removing repression by Gal80TS the dpp-LHG line forces hid activity in the dpp domain (red), located just anterior to the A/P line. At the same time the posterior compartment cells lose the potential to gain JNK activity due to puc over-expression. The results are illustrated in D–F′. Cells of posterior origin (green) can penetrate in the A compartment if they can activate JNK (E–E′′), but are unable to do so if JNK activation is prevented by puc over-expression. Quantitative data are shown in D. Bars represent S.E.M., n = 15 in each genotype, *p<0.05.
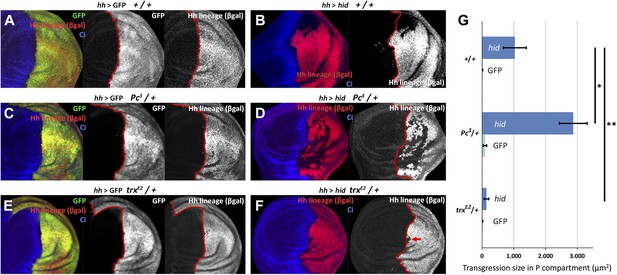
Changes in epigenetic regulation during A/P boundary reconstruction.
Comparison of the size of transgressions after Hid treatment found in discs containing normal doses of Polycomb (Pc) or of trithorax (trx) (A, control with GFP treatment, B experimental), with those in discs containing one dose of Pc (C, control, D experimental) or of trx (E, control, F experimental). Note the significant increase in transgressions size in Pc3/+ background and the reduction in trxE2/+ background (a small transgression is indicated with an arrow in F). Panel G shows the quantification of the transgressions size in the three genotypes, each with respect to its own control. Bars represent S.E.M., n>15 in each genotype, *p<0.01, **p<0.05. See also Figure 5—figure supplements 1 and 2.
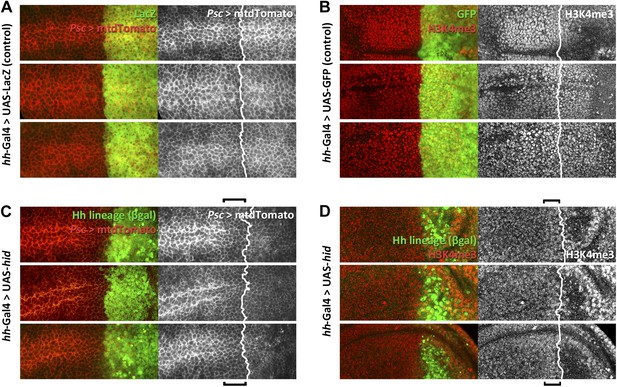
Alterations in the expression of Posterior sex comb (psc) and of H3K4 levels in the proximity of the A/P border after Hid administration.
(A and C). Psc expression (red, mtdTomato) in three control discs is uniform across the border (A), defined by the limit of the lacZ expression under hh control. Note in the three experimental discs (C) a lowering of Psc levels, indicated by the bracket. (B and D) Similar comparison of H3K4 levels between three control (B) and three experimental discs (D). H3K4 is increased in cells in the P compartment and in some anterior cells close to the border, indicated by brackets.
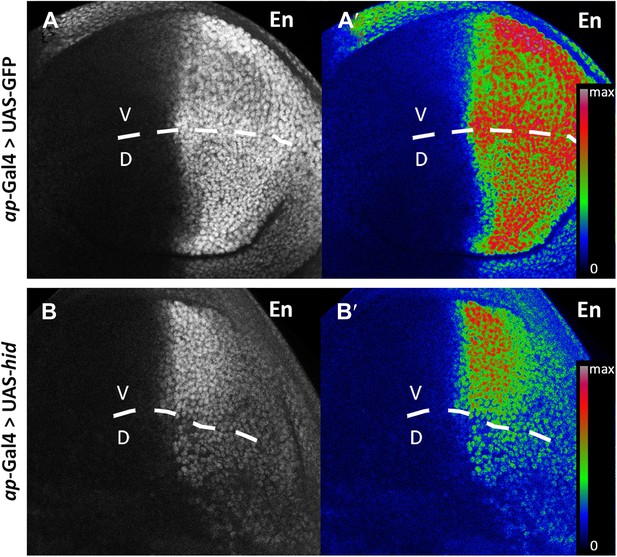
Engrailed protein levels are reduced in posterior cells.
Wing discs after 48 hr of GFP (A–A′, control) or hid (B–B′) induction in the dorsal compartment (apterous-Gal4 domain). A′ and B′ panels show a heat map representation of the Engrailed protein levels. The D/V border is indicated with a discontinuous line. In the hid-expressing disc (B–B′), note the reduction of En levels in the dorsal compared to the ventral compartment.
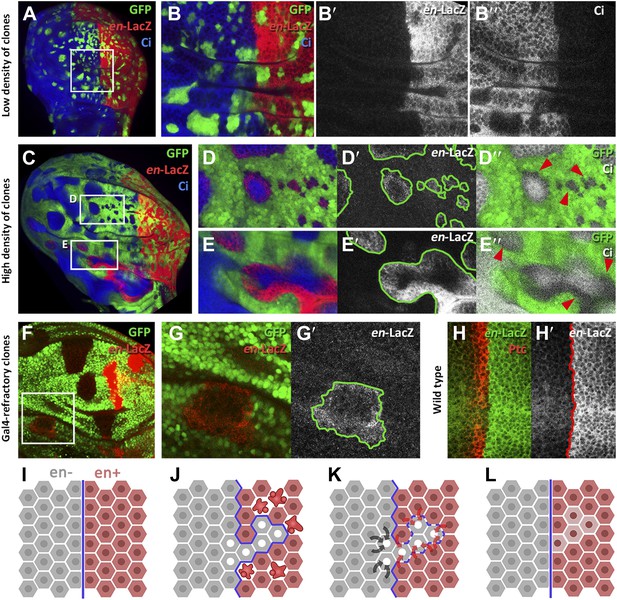
Induction of en activity by en-expressing neighbors.
(A–E′) Clones of cells over-expressing engrailed and GFP were generated 48 hr before puparium formation. The clones of interest are those located in the A compartment. In A compartments with low density of en-expressing clones (A, B–B′′) there is no induction of the en-lacZ reporter in anterior cells, nor there is alteration of Ci levels. However, when the disc is filled with en-expressing clones (C and magnifications in D–E′′) anterior cells in contact with en-expressing clones acquire en-LacZ activity, while Ci protein levels decrease (D′′ and E′′). (F–G′) Disc with Gal80-expressing clones (refractory to Gal4) in the anterior compartment surrounded by cells over-expressing en-LacZ (red) and GFP driven by the act-Gal4 line (see ‘Materials and methods’ for details). The clones show en activity, visualized by en-LacZ (G). (H and H′) High magnification of the A/P border in a control disc doubly stained for Patch, a marker of the A compartment that delineates the A/P border, and en-LacZ. Note that there is no extension of en activity beyond the boundary. (I–L) Scheme of our proposal of the ‘induced by neighbors’ model of en activation during the regeneration of the A/P boundary. Before cell death induction (I) the A and P compartments are separated by a normal straight A/P border. (J) During the cell killing the border collapses due to changes of identity of cells. This allows some intermingling before it is reconstructed; cells of anterior provenance may be surrounded by cells of posterior identity, which induce activity of the endogenous en gene of the anterior cells (K). Once new the identities are established, the differential affinities of the A and P cells contribute to form a new A/P boundary (L). See also Figure 6—figure supplements 1 and 2.
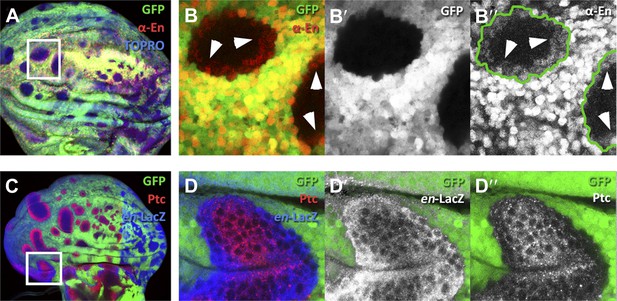
Presence of En protein induced by en-expressing neighbors.
(A–B′′) Clones of en-expressing cells (green) in the A compartment inducing en activity (red) in anterior cells visualized with the anti-En antibody (red). The region of the inset in A is magnified in B–B′′. Notice the En protein in cells close to the border of the clones. (C–D′′) disc containing en-expressing clones (green) stained for en-lacZ (blue) and Patched (red). The inset area is magnified in D–D′′. Notice that the expression of en in anterior cells close to the en-expressing clones (D′) is associated with a diminution of Ptc levels (D′′).
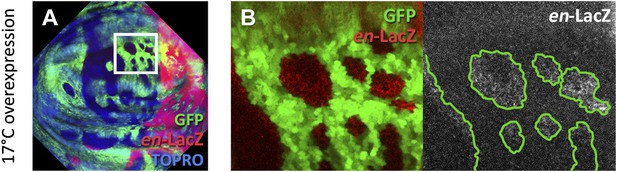
Experiment of induction of clones over-expressing en (green) by the Gal4 system at 17°C.
The genotype of the discs is the same as those in Figure 6; the only difference is the temperature at which the act-Gal4 transgene functions. In the disc in A, the region of the A compartment outlined by the white square is magnified in B. Note the en-lacZ expression (red) in cells outside but close to the borders of en-expressing clones. The A/P border s delimited by the high en-lacZ levels in the P compartment.