Natural antisense transcripts regulate the neuronal stress response and excitability
Figures
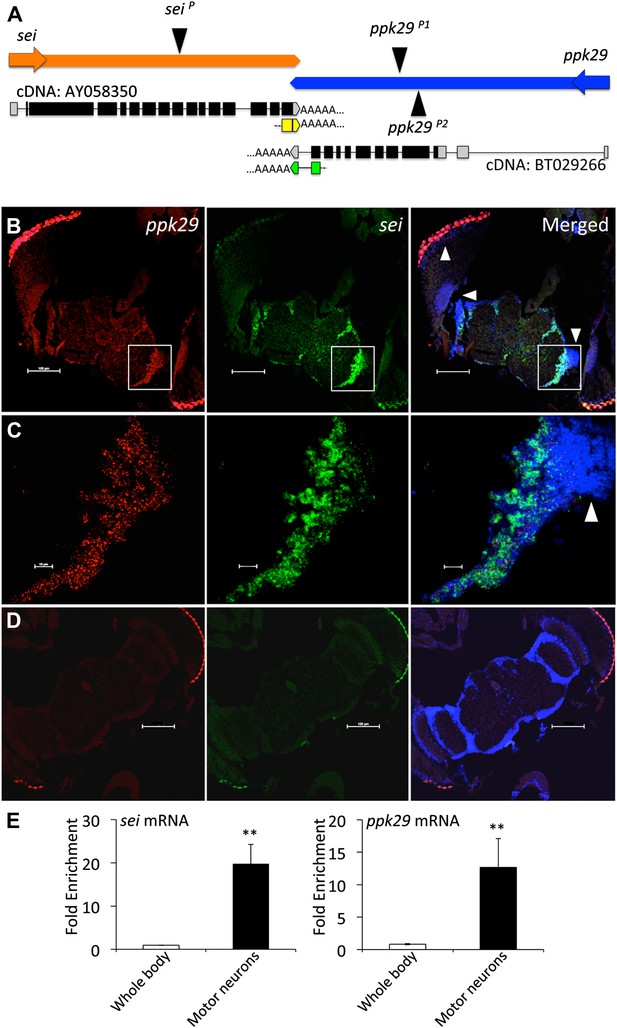
sei and ppk29 are co-expressed in the nervous system.
(A) The chromosomal architecture of sei and ppk29 (2R:19,934,934- 19,944,660). Coding exons are in black. 3′ and 5′ untranslated regions (UTRs) are in gray. AY058350, fully sequenced sei cDNA; BT029266, fully sequenced ppk29 cDNA. Black triangles represent transposons insertion sites. Arrows represent direction of transcription. Yellow boxes, sei 3′RACE product. Green boxes, ppk29 3′RACE product. (B) In situ hybridization shows sei and ppk29 are co-expressed in neuronal tissues. Antisense riboprobes. Scale bar, 100 μm. (C) Higher magnification of white box in B. White arrowheads, optic lobe neurons. Red, ppk29 signal; Green, sei signal; Blue, DAPI nuclear stain. Scale bar, 10 μm. (D) Sense riboprobe controls. Scale bar, 100 μm. (E) Translating Ribosome Affinity Purification (TRAP) of mRNAs from larval motor neurons shows that sei and ppk29 are co-enriched in these cells relative to total body RNA. mRNA levels for each gene were measured with Real-Time qRT-PCR. N = 4 per gene. **p<0.01.
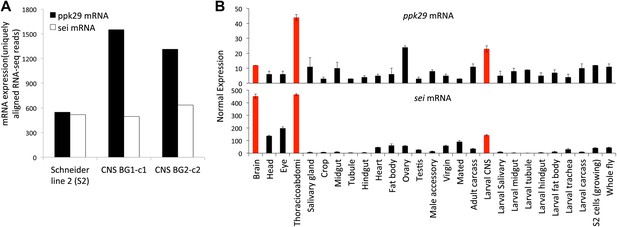
ppk29 and sei are co-expressed in Drosophila neuronal tissues.
(A) ppk29 and sei are co-expressed in neuronal cell lines. Data are from the modEncode database. Expression levels represent average strand-specific unique RNA-seq reads. BG1 and BG2 are neuronal cell lines. Schneider 2 (S2) is an undefined embryonic cell line. (B) Expression of ppk29 and sei in different tissues. Orange bars highlight neuronal tissues. Average data are presented as mean ± SEM (n = 4 arrays per tissue).
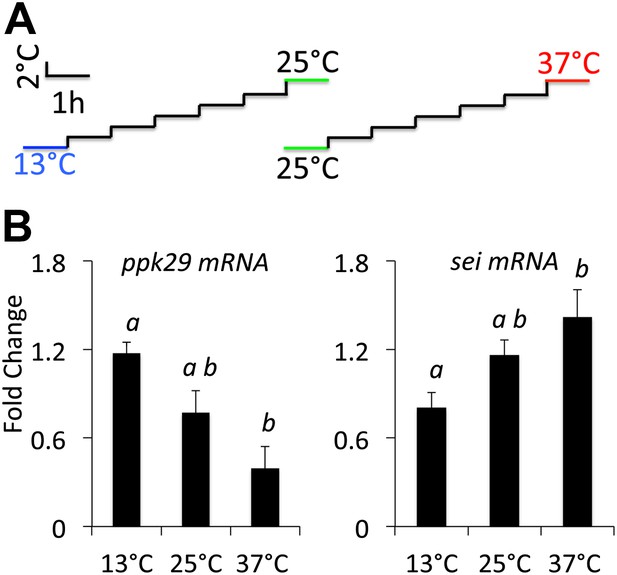
sei and ppk29 transcripts are inversely regulated in response to changes in ambient temperature.
(A) Temperature adaptation protocol. Total time from 25–37°C or 25–13°C is 7 hr. (B) Real-time qRT-PCR data. Different letters above bars represent statistically significant post hoc analyses (Tukey’s, p<0.05, N = 4 per group).
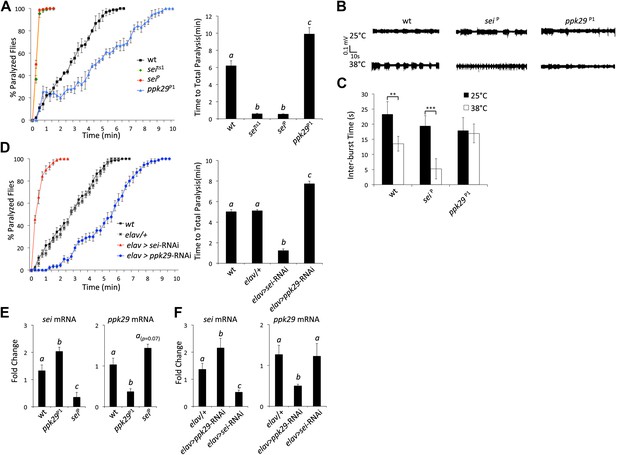
RNAi-dependent knockdowns of ppk29 and sei expression lead to opposing effects on heat-induced paralysis.
(A) The behavioral response to heat stress in sei and ppk29 mutants. Left panel, cumulative paralyzed flies over time. Right panel, same data as in left panel presented as time to total paralysis (n = 16, p<0.001, one-way ANOVA). Different letters above bars represent significantly different groups (Tukey post hoc analysis, p<0.05). (B) Representative extracellular recordings from motor neurons from each genotype at 25°C and 38°C. (C) Summary neurophysiological data (n = 8-10 per genotype, **p<0.01, ***p<0.001, one-way ANOVA with a Tukey post-hoc test). (D) Neuronal downregulation of sei or ppk29 with gene-specific RNAi constructs. Data presented as in A (n = 16, p<0.001, one-way ANOVA). (E) sei and ppk29 mRNA levels in sei and ppk29 mutant lines. Analyses were by relative real-time quantitative RT-PCR analyses. Left panel, sei mRNA. Right panel, ppk29 mRNA (n = 4 per genotype, p<0.05, one-way ANOVA). (F) sei and ppk29 mRNA levels in sei and ppk29 RNAi-knockdown lines. Analyses as in E (n = 4 per genotype, p<0.05, one-way ANOVA). Data are presented as mean ± SEM. Different letters above bars represent significantly different groups (Tukey post hoc analysis, p<0.05).
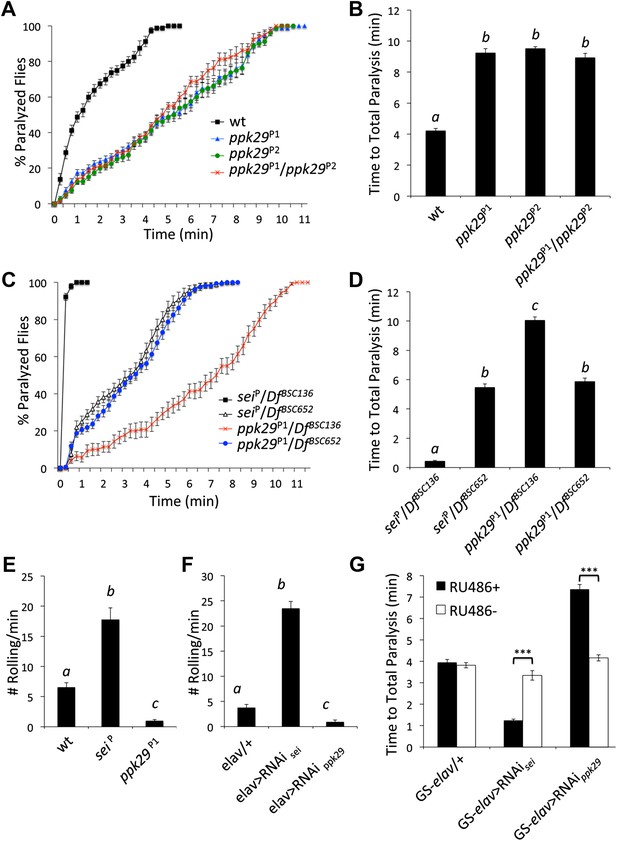
ppk29 mutations confer protection from heat-induced paralysis.
(A) Two independent ppk29 transposon-insertional alleles do not complement each other. Data presented as cumulative paralyzed flies over time. (B) Same data as in A presented as total time to paralysis. Different letters above bars represent significantly different genotypes (one-way ANOVA Tukey's post hoc test; n = 16, p<0.001). (C and D) sei or ppk29 transposon-insertional alleles in trans across a deficiency chromosome (DfBSC136) that covers both loci. Control DfBSC652 has the same genetic background as DfBSC136 but does not cover the sei/ppk29 loci. Analyses and data presentations are as in panels A and B. (E and F) Gene knockdowns of sei or ppk29 by mutations or neuronal RNAi in larvae lead to heat sensitivity or protection phenotypes respectively that are analogous to the adult phenotypes (G) Acute RNAi-dependent targeting of ppk29 or sei in the adult nervous system with the GeneSwitch (GS) version of the pan-neuronal promoter elav was sufficient to phenocopy the mutant phenotypes. Mutant phenotypes were apparent only in the RU486 feeding group (RU486+) (n = 16, ***p<0.001; two-way ANOVA with a Tukey's post-hoc test). The interaction term between genotype and drug was also significant (p=<0.001). Data are presented as mean ± SEM.
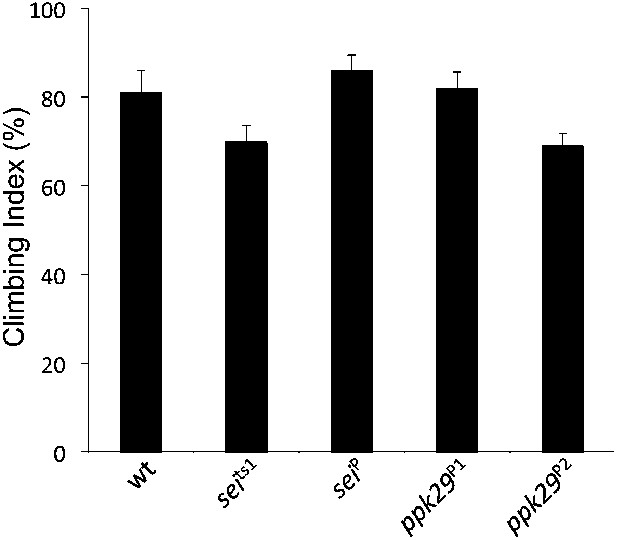
Mutations in sei and ppk29 do not affect gross locomotion at room temperature.
(NS; n = 10 per genotype, one-way ANOVA with Tukey's post-hoc test). Data are presented as mean ± SEM.
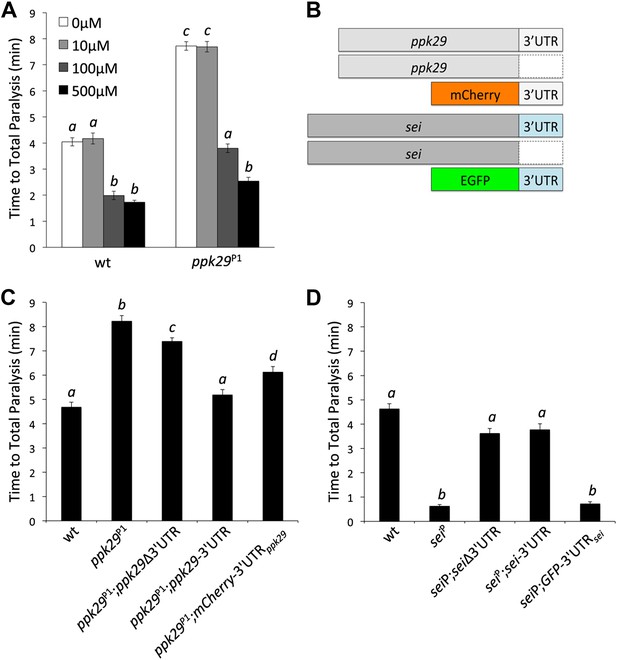
The Protective Effect of ppk29 Mutations is Mediated by SEI Channel Activity.
(A) Blocking SEI channel activity in ppk29 mutants with the hERG channel blocker Cisapride eliminate the protective effect in a dose dependent manner (n = 8 per genotype, p<0.01, two-way ANOVA; genotype, dose, and genotype by dose showed significant effects, p=<0.001). (B) Schematic representation of transgenic constructs. (C) Neuronal expression of ppk29-3′UTR is sufficient to rescue the majority of the protective effect of the ppk29 mutation (n = 12, p<0.01, one-way ANOVA). Data are presented as mean ± SEM. Different letters above bars represent significantly different groups (Tukey post hoc analysis, p<0.05). (D) Neuronal expression of sei cDNA with or without its endogenous 3′UTR, but not the 3′UTR alone, is sufficient to rescue the sei mutation (n = 12, p<0.001, one-way ANOVA).
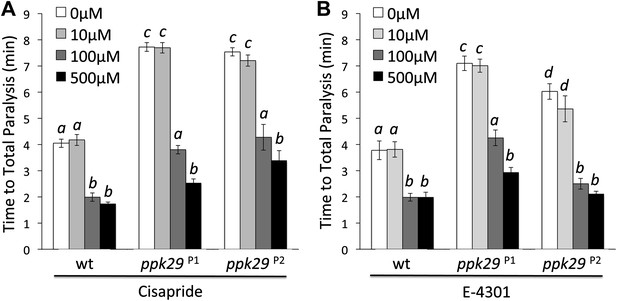
The protective effect of ppk29 mutations depends on SEI K+ channel activity.
Treating ppk29 mutants flies with hERG inhibitors cisapride (A) and E−4301 (B) lead to a significantly faster heat-induced seizures and paralyses in all tested genotypes (n = 8 for each genotype, two-way ANOVA with a Tukey's post-hoc test; the interaction between genotype and concentration is significant for both drugs, p=<0.001). Average data are presented as mean ± SEM. Different letters above bars represent significantly different groups.
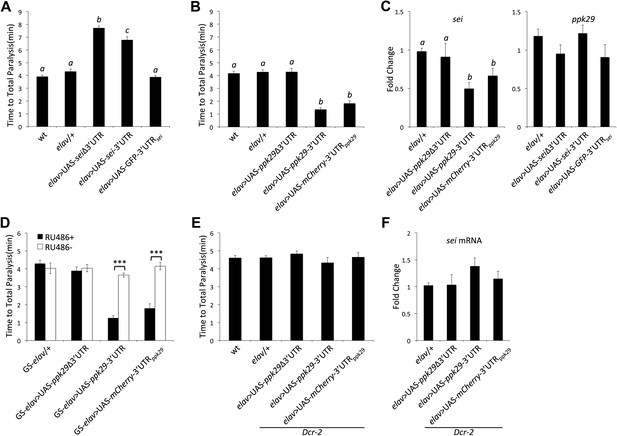
ppk29-dependent regulation of sei depends on the canonical RISC pathway.
(A) Neuronal overexpression of sei cDNA with or without its endogenous 3′UTR in wild type animals leads to a protection from heat-induced paralysis (n = 12, p<0.001, one-way ANOVA). (B) Neuronal overexpression of the ppk29 cDNA with its endogenous 3′UTR or the 3′UTR alone, but not the ppk29 cDNA lone, is sufficient to induce sei mutant-like heat sensitivity phenotype (n = 12, p<0.001, one-way ANOVA). (C) Real-time qRT-PCR analyses of sei and ppk29 mRNA level. Overexpression of ppk29 cDNA with its 3′UTR or the 3′UTR alone, but not the cDNA alone, is sufficient to downregulate endogenous sei mRNA levels (left panel) but not conversely (right panel) (n = 4, p<0.05, one-way ANOVA). (D) Adult-specific neuronal overexpression of ppk29-3′UTR with the hormone inducible GeneSwitch elav-GAL4 is sufficient to induce sei mutant-like phenotype (n = 12, ***p<0.001; two-way ANOVA, genotype, RU486, and their interaction are significant, p=<0.001). (E and F) The effect of ppk29 3′UTR overexpression on heat sensitivity and sei mRNA downregulation is abolished in the Dcr-2 mutant background (n = 12, one-way ANOVA). (F) Real-time qRT-PCR (n = 4, NS, one-way ANOVA). Data are presented as mean ± SEM. Different letters above bars represent significantly different groups (Tukey post hoc analysis, p<0.05).
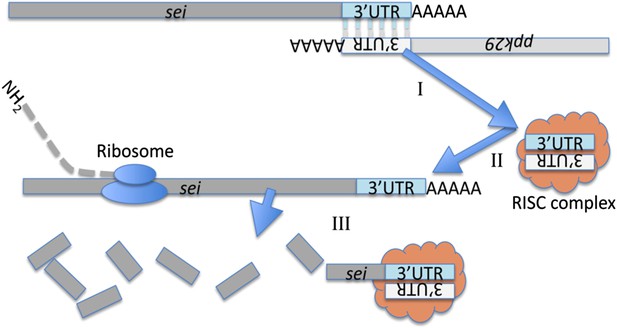
Cartoon depicting a model for the molecular interaction between sei and ppk29.
The chromosomal organization of these two genes suggest they could generate endogenous siRNA by convergent transcription. (I) The complementary 3′UTRs of sei and ppk29 mRNAs form a dsRNA. (II) Dicer-2 cleaves dsRNAs into siRNAs. (III) The loaded RISC complex targets sei transcripts for degradation via the canonical siRNA pathway.
Videos
Wild type larva at 25°C.
seiP larva at 25°C.
ppk29P1 larva at 25°C.
Wild type larva at 38°C.
seiP larva at 38°C.
ppk29P1 larva at 38°C.
Tables
Fly and human eag-like channels that are possibly regulated via convergent transcription with an unrelated mRNA
| Species | eag-like gene | Converging gene |
|---|---|---|
| Drosophila | sei | ppk29 |
| eag | hiw | |
| Human | KCNH1 | HHAT |
| KCNH3 | MCRS1 | |
| KCNH7 | GCA |
-
Please note that the converging genes are functionally diverse, which suggest that their protein identities might not play a role in their regulatory functions.





