Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells
Figures
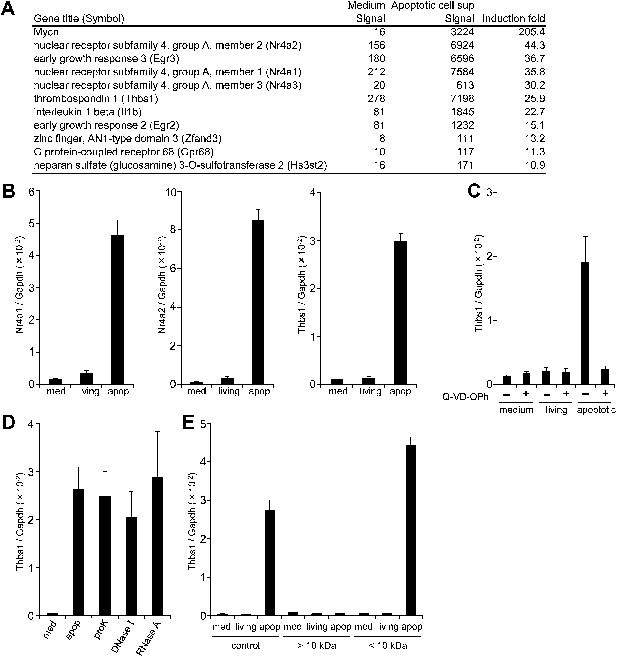
Factor(s) released from apoptotic cells stimulate gene expression in macrophages.
(A and B) BMDMs were incubated for 1 hr with medium or with the supernatant of W3 cells that had been treated with (apoptotic) or without (living) 30 units/ml FasL. RNA from BMDMs was then subjected to microarray analysis. (A) Genes whose expression was upregulated more than 10-fold after incubation with the apoptotic cell supernatant are listed. (B) Nr4a1, Nr4a2, and Thbs1 mRNA levels were quantified by real-time RT-PCR, and normalized to Gapdh mRNA. (C) W3 cells were pre-treated with or without 20 μM Q-VD-OPh for 20 min and stimulated with or without 30 units/ml FasL. BMDMs were then incubated for 1 hr with the supernatant of Q-VD-OPh-treated (+) or untreated (−) living or FasL-treated apoptotic W3 cells, and Thbs1 mRNA levels were determined by real-time RT-PCR. (D) BMDMs were incubated with the supernatant of apoptotic W3 cells that had been treated with proteinase K (proK), DNase I or RNase A, and Thbs1 mRNA levels were determined. (E) Medium, the culture supernatant of healthy W3 cells (living) or apoptotic W3 cells (apop) were subjected to ultrafiltration through a 10 kDa-cutoff filter, and the filtrate (<10 kDa) and concentrate (>10 kDa) were tested for their ability to induce Thbs1 expression in BMDMs. Experiments were performed in triplicates, and the average values are plotted with SD (bars). All experiments were repeated at least twice with BMDM from different mice, and representative data are shown.
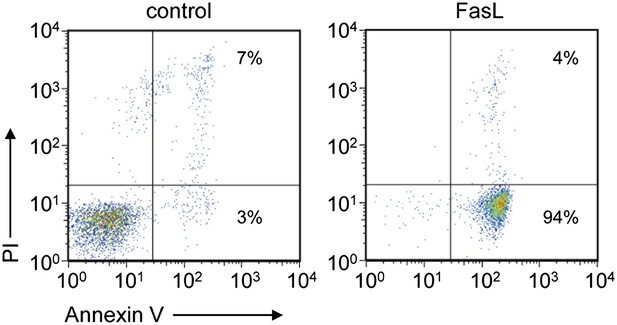
FasL-induced apoptosis in W3 cells.
W3 cells treated with or without 30 units/ml FasL for 90 min were stained with a Cy5-labeled Annexin V and PI and analyzed by flow cytometry. The percentage of positively stained cells in each quadrant is indicated.
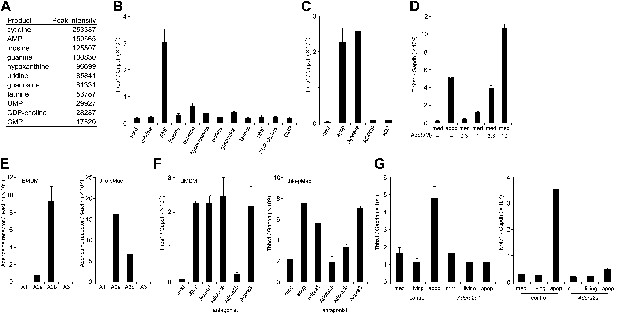
Identification of the factor in apoptotic cell supernatant that stimulates macrophage gene expression.
(A) The supernatant of apoptotic W3 cells was subjected to LC-MS analysis. The relative concentration of each compound is represented by the base peak intensity. (B) BMDMs were incubated with medium containing 10 μM of the indicated reagents for 1 hr, and Thbs1 mRNA levels were determined by real-time RT-PCR. (C) Apoptotic W3 cell supernatant was pretreated with 25 mU/ml apyrase at 37°C for 1 hr. BMDMs were incubated for1 hr with the pretreated or untreated supernatant, and the Thbs1 mRNA levels were determined. BMDMs were also treated with the supernatant in the presence of 10 μM AOPCP or 0.9 U/ml adenosine deaminase (ADA), and the Thbs1 mRNA levels were quantified as above. (D) BMDMs were incubated with apoptotic cell supernatant (apop) or medium supplemented with the indicated concentrations of adenosine (Ado), and the Thbs1 mRNA levels were determined. (E) The mRNA levels of the Adora1, Adora2a, Adora2b, and Adora3 expressed in BMDMs and thio-pMacs were determined by real-time RT-PCR, and normalized to β-actin mRNA. (F) BMDMs and thio-pMacs were incubated with W3 apoptotic cell supernatant and adenosine receptor antagonists, 5 nM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (A1), 10 nM SCH58261 (A2a), 5 μM alloxazine (A2b), or 130 nM VUF5574 (A3), and Thbs1 mRNA levels were determined. (G), Thio-pMacs from Adora2a+/+ (control) or Adora2a−/− mice were incubated with medium, apoptotic or living W3 cell supernatants, and the Nr4a1 and Thbs1 mRNA levels were determined by real-time RT-PCR. Experiments were performed in triplicate, and the average values are plotted with SD (bars). All experiments were repeated at least twice with BMDM or thio-pMacs from different mice, and representative data are shown.
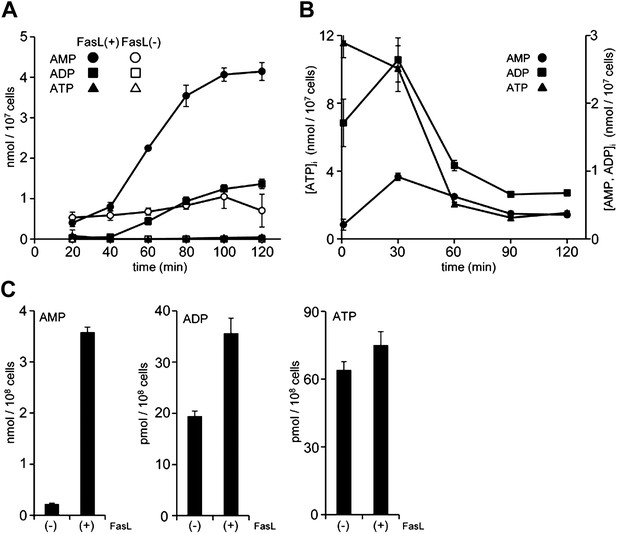
Release of adenine nucleotides from apoptotic cells.
(A and B) W3 cells were treated with (●,■,▲) or without (○,□,△) 120 units/ml FasL, and the concentrations of AMP (○,●), ADP (□,■) and ATP (△,▲) in the supernatant (A) and cells (B) were determined by LC-MS or HPLC analysis at the indicated times. (C) Mouse thymocytes were treated with (+) or without (−) 120 units/ml FasL for 90 min, and the concentrations of AMP, ADP, and ATP in the supernatants were determined. Experiments were performed in triplicate, and the average values are plotted with SD (bars). The treatment of cells with FasL and quantification of adenine nucleotides were repeated at least twice, and representative data are shown.
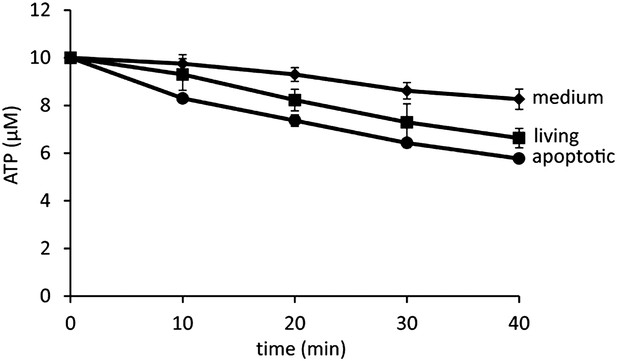
Minimal degradation of ATP in the apoptotic cell supernatant.
ATP was exogenously added to the supernatants of FasL-treated or FasL-untreated W3 cells at a final concentration of 10 μM. At the indicated times, ATP was quantified in triplicate using the luciferase system as described in ‘Materials and methods’, and the average values are plotted with SD (bars).
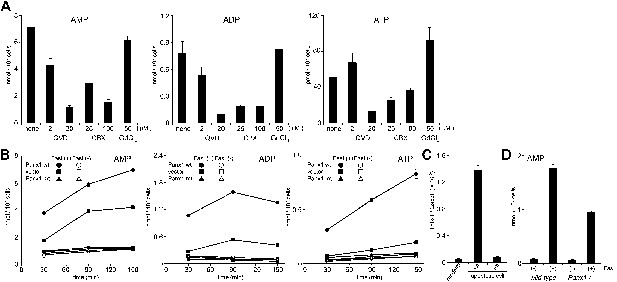
Pannexin 1-dependent release of adenine nucleotides from apoptotic cells.
(A) After pre-treatment with the indicated concentrations of Q-VD-OPh (QVD), carbenoxolone (CBX), or GdCl3 for 20 min, W3 cells were incubated with 120 units/ml FasL for 90 min. The AMP, ADP, and ATP concentrations in the supernatants were determined by LC-MS. (B) W3 cells transformed with empty pMXs vector (■,□), or with vectors bearing wild-type (wt) (●,○) or caspase-resistant mutant (mt) (▲,△) Pannexin 1 were treated (■,●,▲) or not treated (□,○,△) with 120 units/ml FasL, and the concentrations of adenine nucleotides in the supernatants were determined at the indicated times. (C) The parental W3 cells (wt) and W3 cell transformants expressing caspase-resistant form of pannexin 1 (mt) were treated with 30 units/ml FasL for 30 min, added to BMDMs, and incubated for 1 hr. The Thbs1 mRNA level in BMDMs was then determined by real-time RT-PCR. (D) Thymocytes from Panx1+/+ or Panx1−/− mice were incubated with (+) or without (−) 120 units/ml FasL, and the concentration of adenine nucleotides in the supernatants were determined at the indicated times. Experiments were performed in triplicate, and the average values are plotted with SD (bars). All experiments were repeated at least twice, and the representative data are shown.
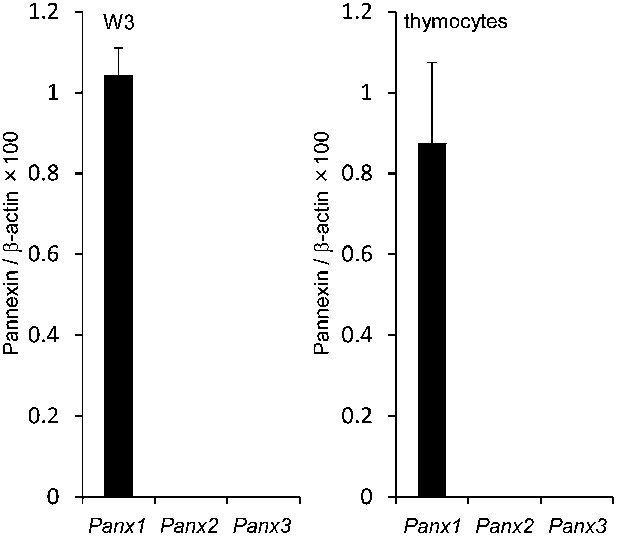
Panx mRNA expression in W3 cells and mouse thymocytes.
Panx1, Panx2, and Panx3 mRNA expression in W3 cells and mouse thymocytes was quantified in triplicate by real-time RT-PCR. The average values for each mRNA are expressed as relative values to β-actin mRNA with SD (bars).
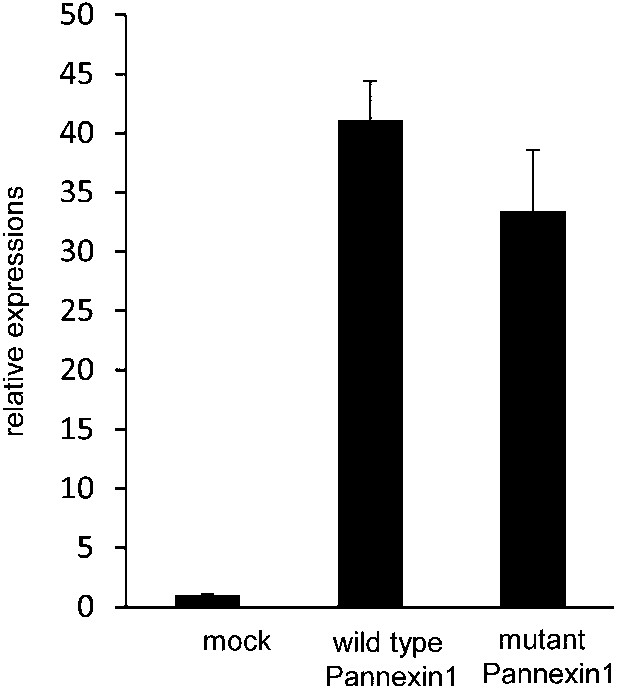
Expression of wild-type and caspase-resistant Pannexin 1 in W3 cell transformants.
Expression plasmids containing wild-type and mutant Panx1 genes were introduced into W3 cells. The Panx1 mRNA levels in each transformant were quantified in triplicate by real-time RT-PCR. The average Panx1 mRNA levels were normalized to the endogenous Panx1 mRNA, and expressed as ‘relative expression’ against the endogenous Panx1 with SD (bars).
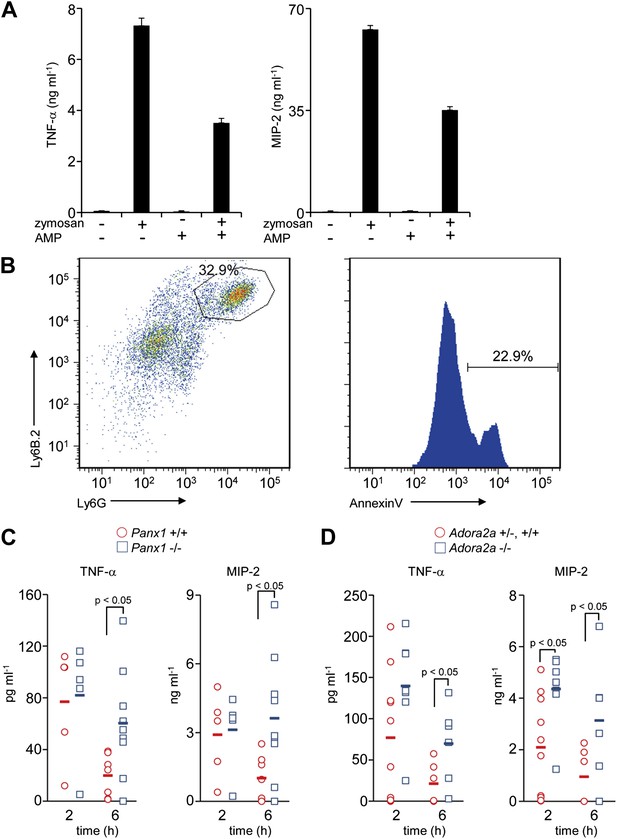
Immunosuppressive function of Pannexin 1 and A2a adenosine receptor in a mouse peritonitis model.
(A) Mouse resident peritoneal macrophages were incubated with 50 μg/ml zymosan A in the presence (+) or absence (−) of 100 μM AMP for 16 hr. The TNFα and MIP-2 levels in the culture supernatant were determined in triplicate by ELISA, and the average values are plotted with S.D. (bars). The experiments were performed at least twice, and representative data are shown. (B) Peritoneal cells were collected at 3 hr after the injection of zymosan A (250 mg/kg), and stained with anti-Ly6B.2 and anti-Ly6G antibodies (left panel). Some of the Ly6B.2 and Ly6G double-positive cells (circled in the left panel) were stained with Cy5-labeled Annexin V (right panel). (C and D) After zymosan injection, peritoneal lavage fluid was collected at 2 hr (n = 5) or 6 hr (n = 7) of Panx1+/+, and at 2 hr (n = 5) or 6 hr (n = 10) of Panx1−/− littermates (C), or 2 hr (n = 10) or 6 hr (n = 6) of Adora2a+/+ or +/−, and at 2 hr (n = 8) or 6 hr (n = 6) of Adora2a−/− mice (D). The TNFα and MIP-2 levels in the fluids were determined by ELISA. The Student’s t test was used for the statistical analysis, and the p values are shown.





