CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection
Figures
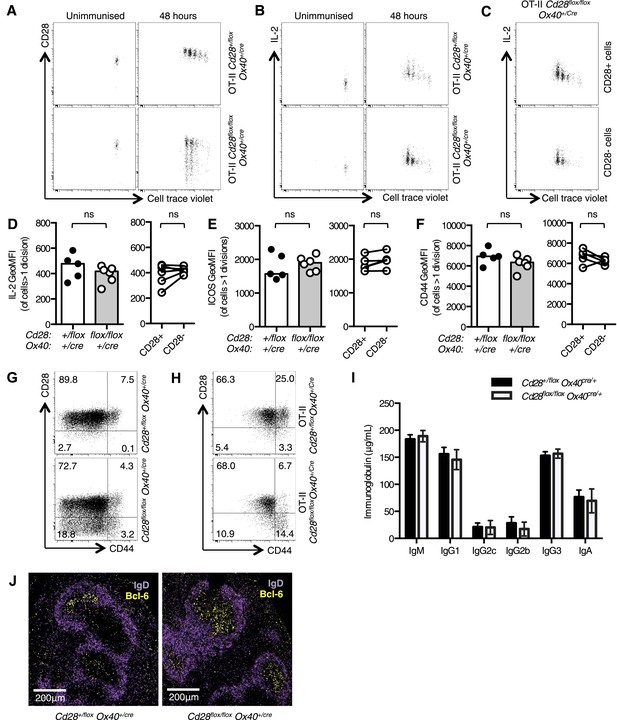
Cd28flox/flox Ox40cre/+ mice lose CD28 expression after T cell priming.
1 × 105 OT-II T cells were labeled with cell trace violet (CTV) and transferred into CD45.1 C57BL/6 hosts and immunized with OVA. The dilution of CTV, CD28 expression (A) and IL-2 production (B) was assessed in OT-II Cd28flox/flox Ox40cre/+ T cells and controls, 48 hr following immunization. The production of IL-2 was also assessed in CD28+ (top panel) and CD28- (lower panel) OT-II cells from OT-II Cd28flox/flox Ox40cre/+mice (C). The expression of IL-2 (D) ICOS (E) and CD44 (F) was quantified for the same experimental system. Flow cytometric dot plots of CD28 and CD44 expression on CD4+ splenocytes from Cd28flox/flox Ox40cre/+ mice (G), and OT-II Cd28flox/flox Ox40cre/+ mice and controls (H). Basal serum immunoglobulins (I) from unmanipulated Cd28flox/flox Ox40cre/+ mice and heterozygous controls. (J) Representative confocal immunofluorescence of IgD (purple) and Bcl-6 (yellow) staining in spleen sections taken 7 days after sheep red blood cell immunization of Cd28+/flox Ox40cre/+ and Cd28flox/flox Ox40cre/+ mice. In D–F heights of the bars represent the median values. In I, J, heights of the bars represent the mean values and error bars represent SEM. For experiments A–F, data are representative of six independent experiments with 5–6 mice per group. Experiments (H and I) are representative of three independent experiments with five mice per group. Experiment (J) is representative of three independent experiments with four mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
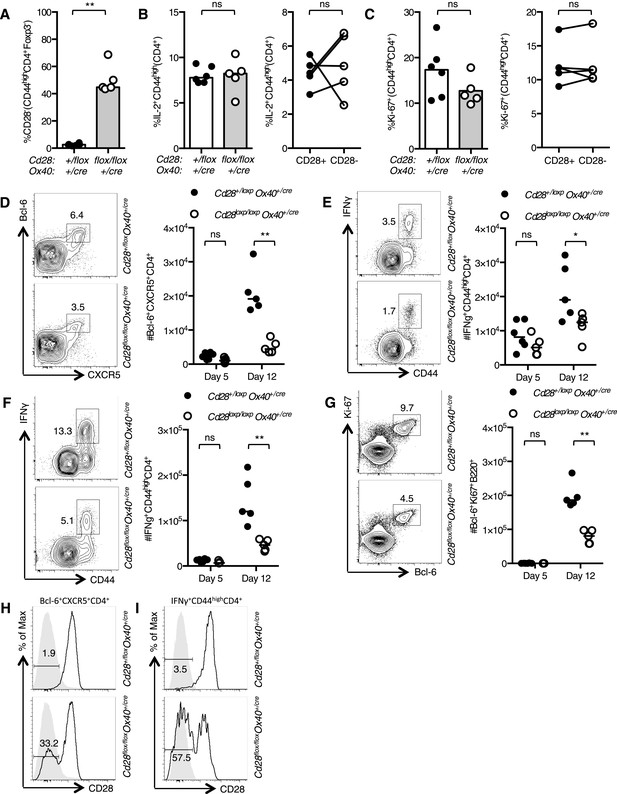
The cellular immune response to Influenza A infection is impaired in Cd28flox/flox Ox40cre/+ mice.
Cd28flox/flox Ox40cre/+ mice and heterozygous controls were infected I.N. with 104 plaque-forming units of influenza A virus. Graphs showing proportion of activated CD4 T cells that have lost CD28 (A) are producing IL-2 (B) and are Ki-67+ (C) in the medLN 5 days post infection. Representative flow cytometric contour plots from day 12 post infection and graphs show the number of medLN Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (D), IFNγ+CD44highCD4+ Th1 cells in the medLN (E) and lung (F) and medLN Bcl-6+Ki67+B220+ germinal center B cells (G) at day 5 and day 12 following infection in Cd28+/flox Ox40cre/+ and Cd28flox/flox Ox40cre/+ mice. Histograms of CD28 expression on medLN Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (H) and lung IFNγ+CD44highCD4+ Th1 cells (I) 12 days after influenza infection. Heights of the lines on graphs represent the median values. Data are representative of three independent experiments with 5–8 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
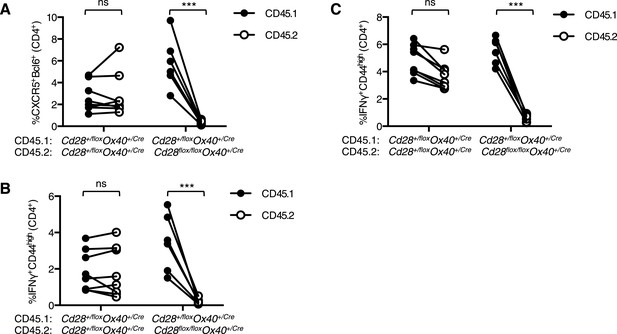
Tfh and Th1 cells from Cd28flox/flox Ox40cre/+ origin are outcompeted in mixed bone marrow chimeras.
Irradiated Rag2−/− mice were reconstituted with a 1:1 ratio of CD45.1 Cd28+/flox Ox40cre/+: CD45.2 Cd28flox/flox Ox40cre/+ bone marrow, and control chimeras with CD45.1 Cd28+/flox Ox40cre/+: CD45.2 Cd28+/flox Ox40cre/+ bone marrow. 8 weeks after reconstitution these chimeras were infected I.N. with influenza A virus and the proportion of medLN Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (A) and medLN (B) and lung (C) IFNγ+CD44highCD4+ Th1 cells 12 days post infection. CD45.1 and CD45.2 cells from the same mouse are connected. Data are representative of two independent experiments with 5–7 mice per group. Ns = not significant, ***p < 0.005.
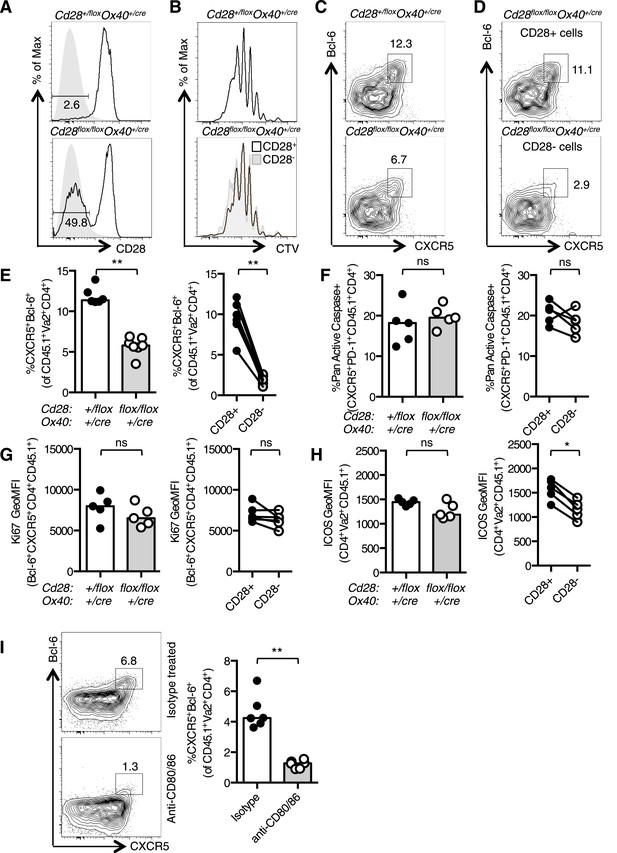
Tfh formation required CD28 signaling after T cell activation.
1 × 105 OT-II T cells were and transferred into CD45.1 C57BL/6 hosts and immunized with OVA I.P. CD28 expression (A) on splenic CD4+Va2+CD45.2+ cells 3.5 days after immunization, gray filled histograms show isotype controls. Dilution of CTV (B) in CD28+ cells (open histograms) and CD28- cells (gray histogram). Bcl-6+CXCR5+CD4+Foxp3− pre-Tfh cells in transferred OT-II Cd28+/flox Ox40cre/+ and OT-II Cd28flox/flox Ox40cre/+ cells (C) and in CD28+ and CD28- cells (D) from OT-II Cd28flox/flox Ox40cre/+ cells. Proportion of Bcl-6+CXCR5+CD4+Foxp3- pre-Tfh cells (E), percentage of Pan-Active Caspase+ pre-Tfh (F), expression of Ki-67 in pre-Tfh (G) and ICOS on pre-Tfh (H) from transferred OT-II Cd28+/flox Ox40cre/+ and OT-II Cd28flox/flox Ox40cre/+ cells (left graphs) and in CD28+ and CD28- cells from OT-II Cd28flox/flox Ox40cre/+ cells (right graphs). Paired samples are connected with a line. (I) Pre-Tfh 4 days after transfer of 1 × 105 OT-II T cells into CD45.2 C57BL/6 hosts immunized with OVA I.P. followed by CD80/86 blocking antibodies I.V. 64 hr following immunization. Heights of the bars represent the median values. A–H, data are representative of four experiments with 5–7 mice per group. I is representative of three experiments with six mice per group. Ns = not significant, **p < 0.005, ***p < 0.005.
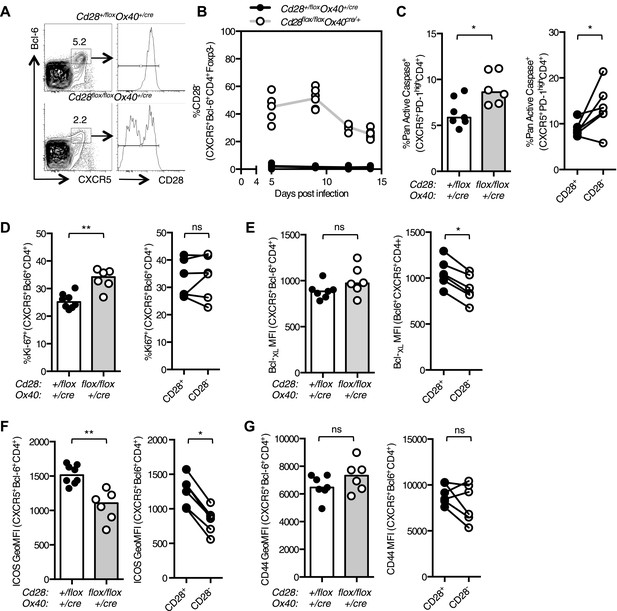
Maintained CD28 expression is required for the maintenance of Tfh cells during influenza infection.
Cd28flox/flox Ox40cre/+ mice and heterozygous controls were infected I.N. with 104 plaque-forming units of influenza A virus. The percentage of Bcl-6+CXCR5+CD4+Foxp3- Tfh cells that lose CD28 expression was quantified at days 5, 9, 12, and 14 post infection in the medLN (A and B). The proportion of Pan Active caspase+ Tfh cells (C), Ki-67+ Tfh cells (D), the expression of Bcl-XL (E), ICOS (F) and CD44 (G) (%CD44+ mean 92.7% for Cd28+/flox Ox40cre/+ and 89.1% for Cd28flox/flox Ox40cre/+) on Tfh cells were enumerated at d14 post infection, comparing between Cd28flox/flox Ox40cre/+ mice and heterozygous controls (left graphs) and also between CD28+ and CD28- Tfh cells (right graphs) from Cd28flox/flox Ox40cre/+ mice. Paired samples from the same mouse are connected with a line and heights of the bars in bar graphs represent the median value. Data are representative of four independent experiments with 5–7 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
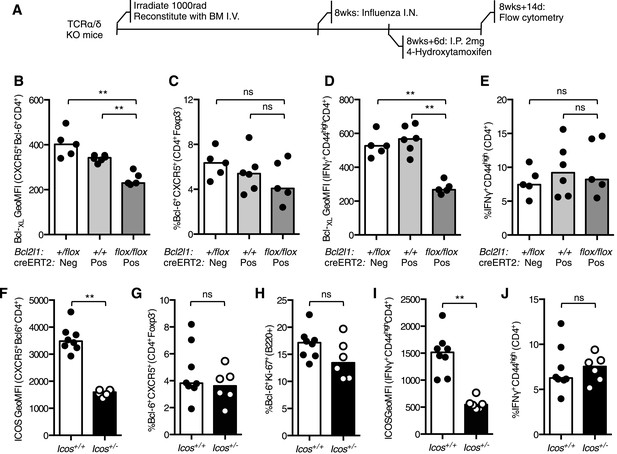
Reduced Bcl-XL or ICOS expression does not impair the Tfh or Th1 population during influenza infection.
(A) TCRα/δ-deficient mice were irradiated and reconstituted with 2 × 106 bone marrow cells of the following combinations: Bcl2l1+/flox:creERT2−, Bcl2l1+/+:creERT2+, and Bcl2l1flox/flox:creERT2+. 8 weeks following reconstitution, the mice were infected with influenza A virus I.N., and 6 days following infection, the mice were treated with 4-hydroxytamoxifen to induce cre expression. The expression of Bcl-XL within medLN Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (B) and their proportions (C) and Bcl-XL expression in lung IFNγ+CD44highCD4+ Th1 cells (D) and their proportions (E) were measured by flow cytometry. Heights of the bars represent the median values. Data are representative of three independent experiments with 5–6 mice per group. Icos+/− mice and wild-type control mice were infected I.N. with influenza A virus and the expression of ICOS on medLN Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (F), the proportion of medLN Tfh cells (G) and germinal center Bcl-6+Ki67+B220+ (H) B cells were determined. The expression of ICOS on lung IFNγ+CD44highCD4+ Th1 cells (I) proportion of these cells (J) was measured by flow cytometry. Heights of the bars represent the median values. Data are representative of two independent experiments with 6–8 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
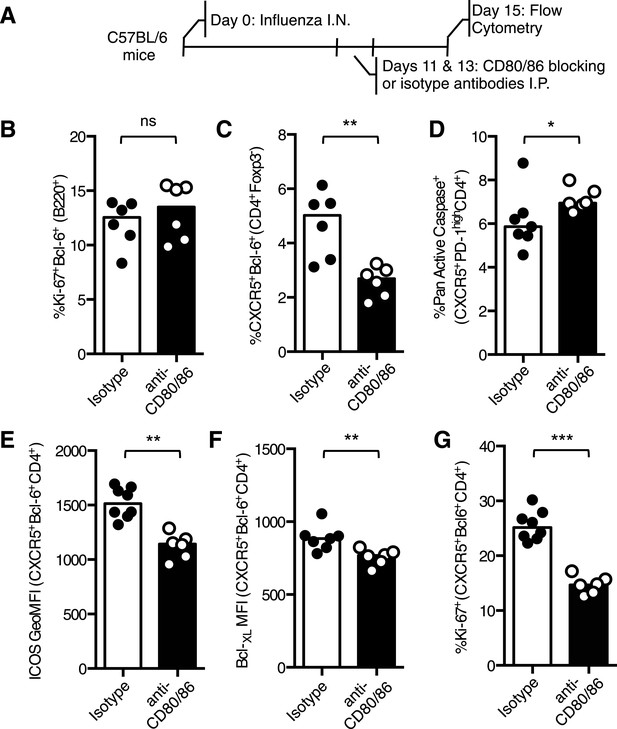
Blocking CD28 ligands reduced Tfh cells in an established infection.
(A) C57BL/6 mice were infected I.N. with influenza A virus and administered CD80/86 blocking antibodies I.P. at days 11 and 13 post infection. The populations of medLN germinal center Bcl-6+Ki67+B220+ B cells (B) and medLN Bcl-6+CXCR5+CD4+Foxp3- Tfh cells (C) were measured by flow cytometry. The percentage of apoptotic pan active caspase+ Tfh cells (D), expression of ICOS (E) and Bcl-XL (F), and proportion of proliferating Ki67+ Tfh cells (G) on Tfh cells was assessed by flow cytometry. Heights of the bars represent the median values. Data are representative of three independent experiments with 6–7 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
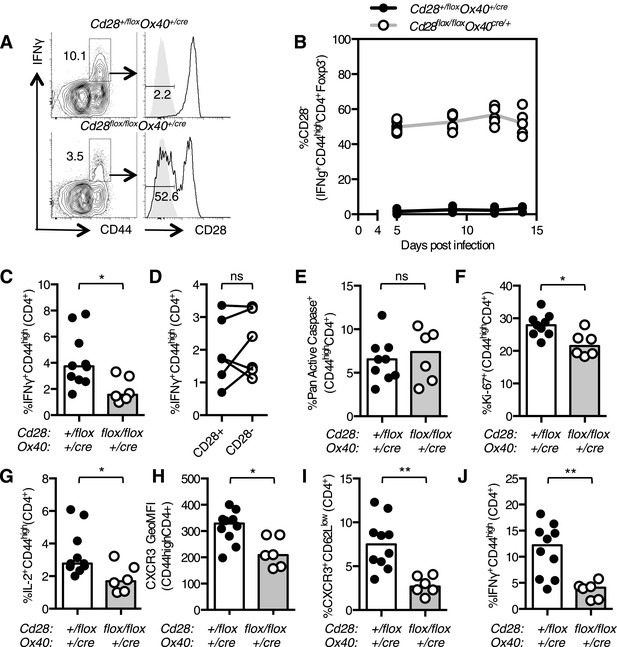
Th1 expansion requires maintained CD28 signaling.
Cd28flox/flox Ox40cre/+ mice and heterozygous controls were infected I.N. with 104 plaque-forming units of influenza A virus. The percentage of IFNγ+CD44highCD4+ Th1 cells that lose CD28 expression was quantified at days 5, 9, 12, and 14 post infection in the lung (A and B). The proportion of IFNγ+CD44highCD4+ Th1 cells (C, D), apoptotic Pan Active Caspase+CD44highCD4+ cells (E), proliferating Ki-67+CD44highCD4+ cells (F), IL-2+CD44highCD4+ cells (G), expression of CXCR3 on CD44highCD4+ cells (H), the proportion of CXCR3+CD62LlowCD4+ (I) cells were assessed in the medLN 7 days post infection. The percentage of lung IFNγ+CD44highCD4+ Th1 cells (J) was also assessed at the same time point. In D, paired samples from the same mouse are connected with a line and heights of the bars in bar graphs represent the median value. Data are representative of three independent experiments with 5–10 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
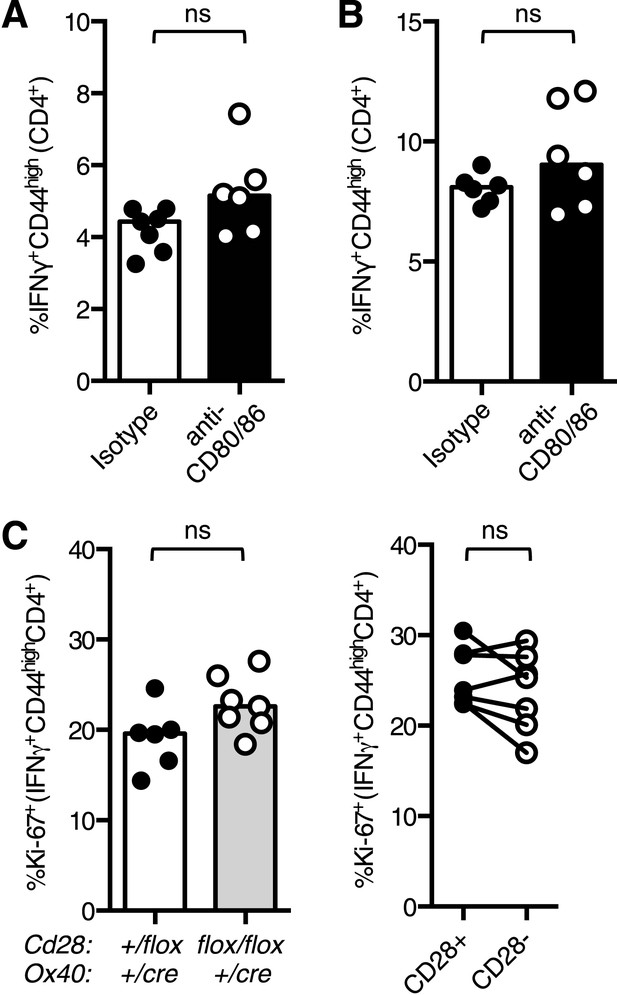
Maintenance of lung Th1 cells is independent of CD28 expression.
(A and B) C57BL/6 mice were infected I.N. with influenza and administered CD80/86 blocking antibodies I.P. at days 11 and 13 post infection. The proportion of CD4+ cells that formed IFNγ+CD44highCD4+ Th1 cells in the medLN (A) and lung (B) were ascertained by flow cytometry. The proportion of Ki-67+ Th1 cells (C) were enumerated at d14 post infection, comparing Cd28flox/flox Ox40cre/+ mice and heterozygous controls (left graphs) and also between CD28+ and CD28- Tfh cells (right graphs) from Cd28flox/flox Ox40cre/+ mice. In C, paired samples from the same mouse are connected with a line and heights of the bars in bar graphs represent the median value. Data are representative of three independent experiments with 5–10 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
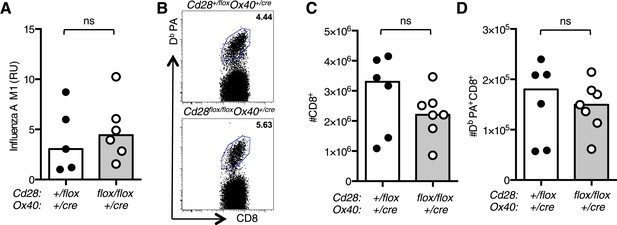
Impaired CD4+ effector T cell responses do not affect resolution of influenza infection.
(A) RNA encoding the influenza A virus M1 protein was determined in the lung 7 days post infection by qRT-PCR. Each dot represents the mean expression of three technical replicates per mouse. (B) Representative flow cytometric dot plots of CD8 cells binding the influenza epitope PA224-233 as identified by Db PA-APC dextramer 12 days after influenza A infection. Total CD8+ cells (C) and Db PA dextramer binding CD8+ cells (D) are enumerated in bar graphs. Each symbol represents one mouse and heights of the bars show the median values. Data are representative of two independent experiments with 5–6 mice per group. ns = not significant.
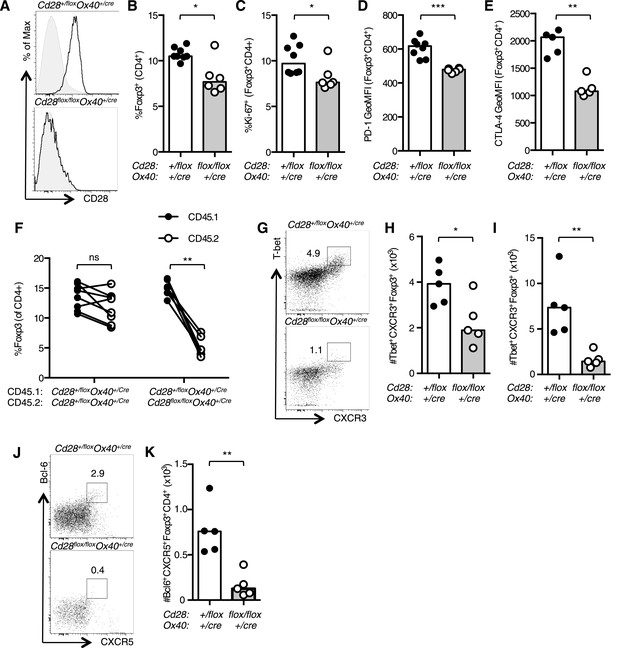
CD28 is required for Treg differentiation.
Histograms of CD28 expression on Foxp3+CD4+ splenocytes (A) from Cd28flox/flox Ox40cre/+ mice and heterozygous controls. Proportion of Foxp3+ Tregs within the CD4+ splenic T cell population (B). Percentage of proliferating Ki-67+ Tregs (C) and expression of PD-1 (D) and CTLA-4 (E) on splenic Tregs. (F) Regulatory T cells from mixed bone marrow chimeras of specified genotypes 12 days post influenza A virus infection. (G–K) Cd28flox/flox Ox40cre/+ mice and heterozygous controls were infected I.N. with 104 plaque-forming units of influenza A virus and 14 days following influenza infection the percentage and number of Tbet+CXCR3+Foxp3+CD4+ cells in the medLN (G and H), lung (I), and Bcl-6+CXCR5+Foxp3+CD4+ Tfr cells in the medLN (J and K) were assessed by flow cytometry. The heights of the bars represent median values. Data are representative of three independent experiments with 5–8 mice per group. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.
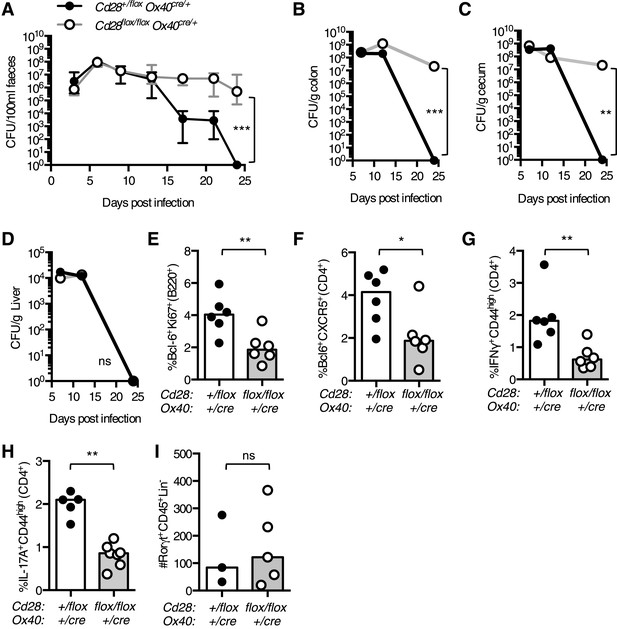
Maintained CD28 expression is required for clearance of C. rodentium.
Cd28flox/flox Ox40cre/+ mice and heterozygous controls were infected orally with C. rodentium and CFU/100 ml of feces was determined at 3–4 day intervals for 24 days (A). Bacterial load in the colon (B), cecum (C), and liver (D) was enumerated 7, 12 and 24 days post infection. 12 days post infection, the percentage of Bcl-6+Ki-67+B220+ germinal center B cells (E), Bcl-6+CXCR5+CD4+Foxp3− Tfh cells (F) and IFNγ+CD44highCD4+ Th1 cells (G) was assessed in the mesenteric lymph node. The percentage of IL-17A+CD44highCD4+ Th17 cells was assessed in the mesenteric lymph node (H), and the number of Rorγt+CD45+Lin− ILC3 cells was assessed in the colon (I) 7 days post infection. The heights of the bars represent median values. In A, the error bars show the range. In B–D, the error bars show SEM. Ns = not significant, *p < 0.05, **p < 0.005, ***p < 0.005.





